Working groups
KONG LAB
The Kong Lab focuses on advancing the understanding and treatment of pancreatic ductal adenocarcinoma (PDAC) through innovative research in several key areas:
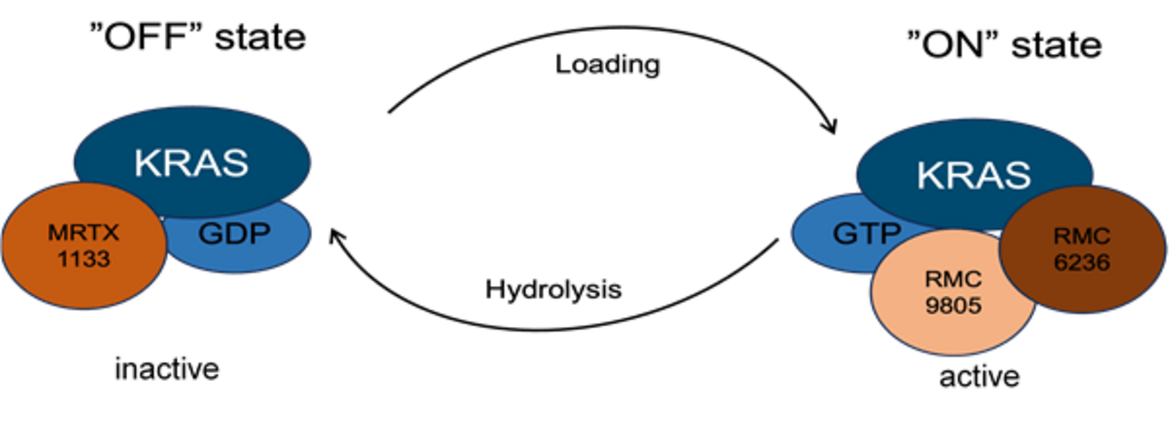
KRAS Inhibition
We use a variety of preclinical models to replicate human PDAC, aiming to predict its response to KRAS inhibition.
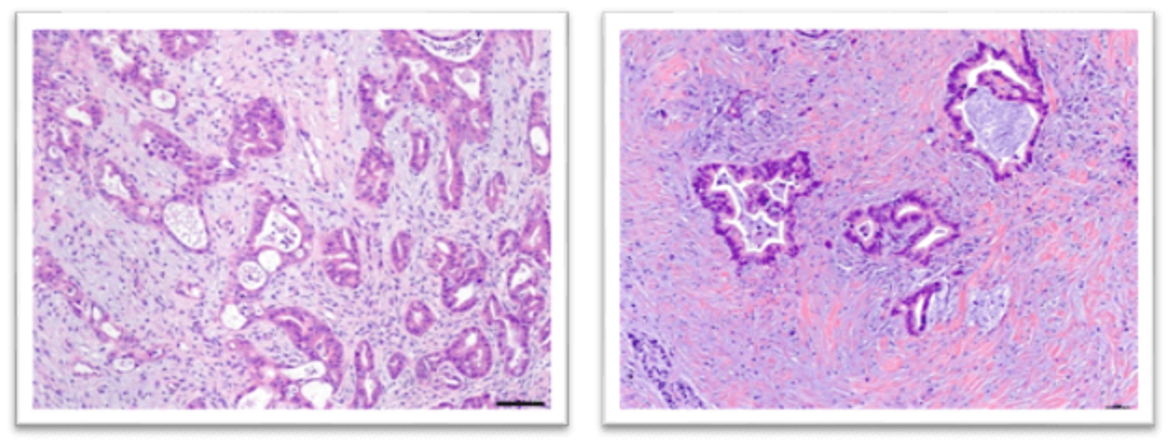
In Vivo Models of PDAC
A significant focus of our laboratory is the development of transgenic mouse models that reflect both the molecular and histological diversity of PDAC. By modifying the cell of origin and genetic makeup, we create multiple mouse models that emulate characteristic features of human PDAC, including desmoplastic reactions and immune-exclusion phenotypes.
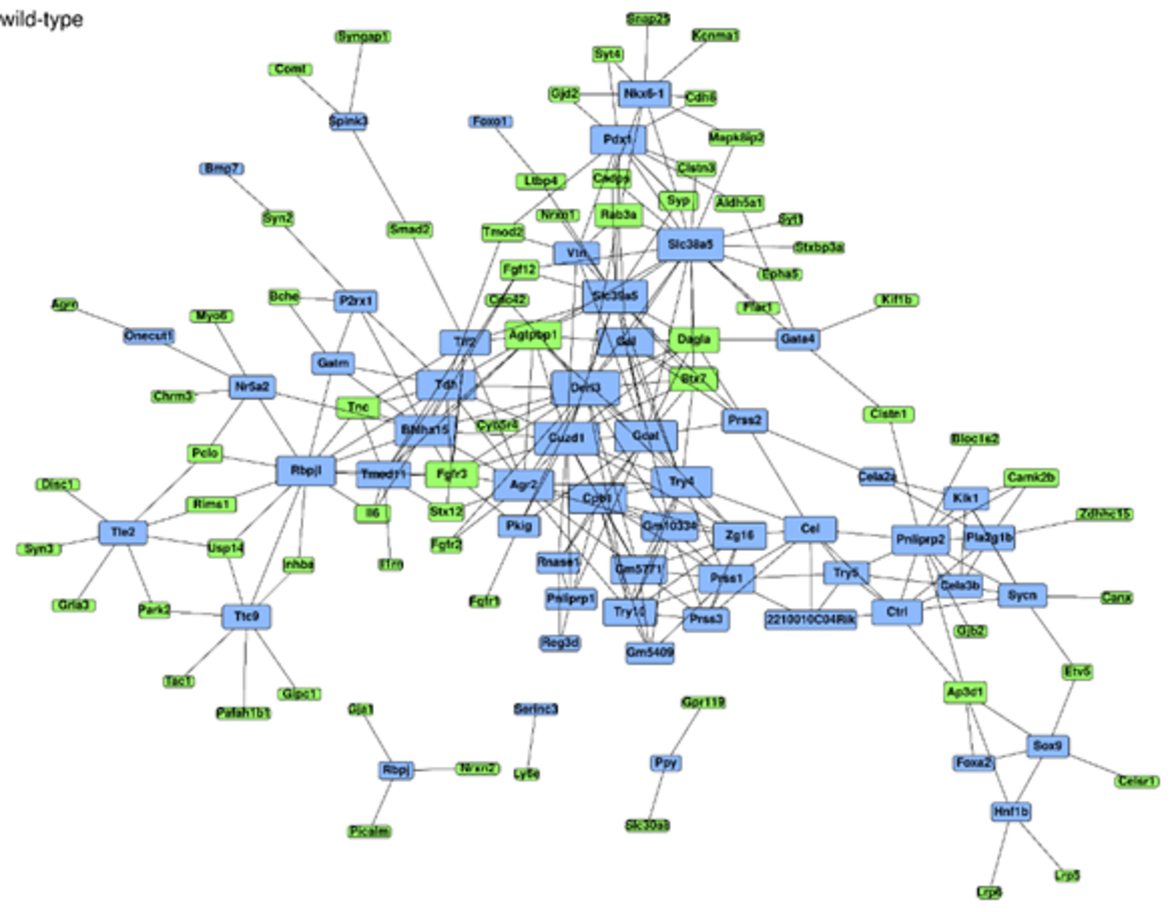
Cancer Signal Simulation
Our team is dedicated to simulating alterations in cancer signalling during pancreatic organ regeneration and carcinogenesis. We concentrate particularly on the dynamics of KRAS and mTOR signalling pathways in PDAC development, with a major emphasis on the adaptive changes following KRAS and mTOR inhibition.
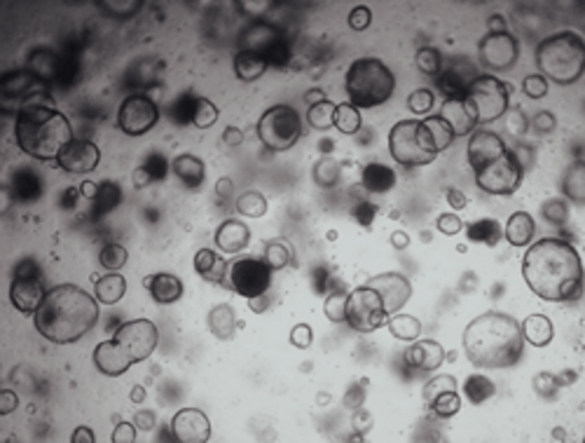
Patient-Derived PDAC Models and Therapeutic Testing
We are developing advanced patient-derived PDAC models that can be readily used to predict potential therapeutic responses. These individualised three-dimensional organoid models allow us to mimic the complexities of PDAC, offering a unique platform for studying therapeutic responses.
Working group leader
Team
Team Leader
PD Dr. Dr. med. Bo Kong
Head of scientific editorial team
Prof. Dr. rer. nat. Ingrid Herr
Senior scientists and advisers
PD Dr. Franco Fortunato (PhD)
Dr. rer. nat. Michael Schäfer
Post Docs
Dr. sc. hum. Bindhu Madhavan
Yina Qiao
Kai Hu
Technicians
Sonja Bauer (maternity leave)
Mohamad Freigeh (maternity cover, as of June 1, 24)
Elvira Mohr
Sascha Hinterkopf
Doctoral candidates
Chao Fang
Jingxiong Hu
Xiaoyan Huang
Yiqi Niu
Lingling Zhang
Qing Zhen
Yiping Yin
Zhaiyue Xu
Enis Salihi
Zeynep Dal
Britta Statz
Franz Li (HIWI/student asssistant)
Secretary
Ellen Watson
Financing
Third-party funds
Selected publications
2025-Cell Reports Medicine
Disrupting AGR2/IGF1 paracrine and reciprocal signaling for pancreatic cancer therapy
Li H, Zhang Z, Shi Z, Zhou S, Nie S, Yu Y, Zhang L, Sun Y, Fang C, Hu J, Niu Y, Schuck K, Wang L, Jiang K, Lu Z, Kahlert C, Roth S, Loos M, Herr I, Sunami Y, Kleeff J, Friess H, Reichert M, Dantes Z, Zou X, Michalski CW, Shen S, Kong B.
2025-International Journal of Cancer
Sun Y, Qiao Y, Niu Y, Madhavan BK, Fang C, Hu J, Schuck K, Traub B, Friess H, Herr I, Michalski CW, Kong B.
2021-Gastroenterology
Zhang Z, Li H, Deng Y, Schuck K, Raulefs S, Maeritz N, Yu Y, Hechler T, Pahl A, Fernández-Sáiz V, Wan Y, Wang G, Engleitner T, Öllinger R, Rad R, Reichert M, Diakopoulos KN, Weber V, Li J, Shen S, Zou X, Kleeff J, Mihaljevic A, Michalski CW, Algül H, Friess H, Kong B.
2021-Gastroenterology
Zhao Y, Schoeps B, Yao D, Zhang Z, Schuck K, Tissen V, Jäger C, Schlitter AM, van der Kammen R, Ludwig C, D'Haese JG, Raulefs S, Maeritz N, Shen S, Zou X, Krüger A, Kleeff J, Michalski CW, Friess H, Innocenti M, Kong B.
2019- International Journal of Cancer
Cheng T, Zhang Z, Jian Z, Raulefs S, Schlitter AM, Steiger K, Maeritz N, Zhao Y, Shen S, Zou X, Ceyhan GO, Friess H, Kleeff J, Michalski CW, Kong B.
2018- Cellular and Molecular Gastroenterology and Hepatology
Jian Z, Cheng T, Zhang Z, Raulefs S, Shi K, Steiger K, Maeritz N, Kleigrewe K, Hofmann T, Benitz S, Bruns P, Lamp D, Jastroch M, Akkan J, Jäger C, Huang P, Nie S, Shen S, Zou X, Ceyhan GO, Michalski CW, Friess H, Kleeff J, Kong B.
2018 - Gut
Dynamic landscape of pancreatic carcinogenesis reveals early molecular networks of malignancy.
Kong B, Bruns P, Behler NA, Chang L, Schlitter AM, Cao J, Gewies A, Ruland J, Fritzsche S, Valkovskaya N, Jian Z, Regel I, Raulefs S, Irmler M, Beckers J, Friess H, Erkan M, Mueller NS, Roth S, Hackert T, Esposito I, Theis FJ, Kleeff J, Michalski CW.
2016-Oncogenesis
In vivo functional dissection of a context-dependent role for Hif1α in pancreatic tumorigenesis.
Cheng T, Jian Z, Li K, Raulefs S, Regel I, Shen S, Zou X, Ruland J, Ceyhan GO, Friess H, Michalski CW, Kleeff J, Kong B.
2016- Gut
Kong B, Wu W, Cheng T, Schlitter AM, Qian C, Bruns P, Jian Z, Jäger C, Regel I, Raulefs S, Behler N, Irmler M, Beckers J, Friess H, Erkan M, Siveke JT, Tannapfel A, Hahn SA, Theis FJ, Esposito I, Kleeff J, Michalski CW.
2015-Molecular Cancer
Kong B, Cheng T, Qian C, Wu W, Steiger K, Cao J, Schlitter AM, Regel I, Raulefs S, Friess H, Erkan M, Esposito I, Kleeff J, Michalski CW.
2015- International Journal of Surgery
Metabolism gene signatures and surgical site infections in abdominal surgery.
Kong B, Bruns P, Raulefs S, Rieder S, Paul L, Prazeresda Costa O, Buch T, Theis FJ, Michalski CW, Kleeff J.
2011- Nature Reviews Gastroenterology & Hepatology
From tissue turnover to the cell of origin for pancreatic cancer.
Kong B, Michalski CW, Erkan M, Friess H, Kleeff J.
2010-Oncogene
Kong B, Michalski CW, Hong X, Valkovskaya N, Rieder S, Abiatari I, Streit S, Erkan M, Esposito I, Friess H, Kleeff J.







