Arbeitsgruppe Prof. Dr. J. Hassel
Prof. Dr. med. Jessica Hassel
Arbeitsgruppenleiterin
(AG Prof. Dr. J. Hassel)
Research Focus
As clinical scientists our main research focus is the better understanding of patients’ treatments. In the last years especially new immunotherapeutic treatment options with so called immune checkpoint blockers give new hope to be able to control the tumor long-term, especially in patients with a metastasized melanoma. However, only a portion of patients definitely profits from this treatment and besides tumor load measured by serum lactate dehydrogenase levels no good clinical biomarker is available to predict response. Recent publications revealed that neoantigens of the tumor might be one of the main factors to lead to recognition by the immune system and tumor defence. We aim to find biomarkers to be able to predict treatment response and learn from them how the treatments work and why tumors are resistant. This might guide ideas for treatment combination with the intention of overcoming the resistance and enhancing tumor response. In an ex vivo system we aim to test different treatment combinations preclinically to then be able to plan promising treatment combinations in clinical trials.
Projects
cMET as therapeutic target for metastatic uveal melanoma patients
Uveal melanoma (UM) is a relatively rare tumor, with just 5 or 6 cases per million people per year. However, it is the most common primary eye tumor and becomes deadliest when it spreads to other parts of the body, especially to the liver. Immunotherapies like immune checkpoint inhibitors (ICIs) are of limited success for metastatic uveal melanoma (mUM). In January 2022, for the first time, the US Food and Drug Administration approved tebentafusp (KIMMTRAK) for mUM treatment. Tebentafusp improved mUM patients' survival; However, only about 50 percent of patients who are positive for the HLA-A*02:01 gene are eligible for the treatment, and tumor response rates are low. Hence, further research is required to investigate potential UM targets that induce tumor death and stimulate anti-tumor immune responses.
In this regard, cMET, a tyrosine kinase receptor that is highly expressed in uveal melanomas and involved in liver metastasis of UM, could be promising. We aim to investigate the potential of cMET inhibitors, especially for their ability to increase anti-tumor immune responses to boost immunotherapy efficacy; possibly leading to new immunotherapeutic combinatorial approaches for mUM patients in the future.
Funding: Hiege-Stiftung Research Grant
Expression and role of the immune checkpoints LAG-3 and TIM-3 in melanoma
Immunotherapies targeting the immune checkpoints CTLA-4 and PD-1 greatly improved survival of melanoma patients. In addition, the antibody relatlimab, which targets the immune checkpoint LAG-3 was clinically approved in 2022 in combination with nivolumab (αPD-1) for treatment of unresectable metastatic melanoma. However, only a subset of patients responds to immune checkpoint blockade. Investigating the role of newly emerging immune checkpoints as LAG-3 and TIM-3 and the factors that influence immunotherapy response are thus important tasks for research.
While most studies focus on LAG-3 and TIM-3 expression on immune cells, our group observed expression of LAG-3 and TIM-3 in tumour cells of melanoma metastases. We aim to investigate melanoma cell intrinsic LAG-3 and TIM-3 expression using various methods, correlate this data with clinical data and study the functional role of LAG-3 expression in melanoma cells using in vitro model systems.
Immunotherapies targeting the immune checkpoints CTLA-4 and PD-1 greatly improved survival of melanoma patients. In addition, the antibody relatlimab, which targets the immune checkpoint LAG-3 was clinically approved in 2022 in combination with nivolumab (αPD-1) for treatment of unresectable metastatic melanoma. However, only a subset of patients responds to immune checkpoint blockade. Investigating the role of newly emerging immune checkpoints as LAG-3 and TIM-3 and the factors that influence immunotherapy response are thus important tasks for research.
While most studies focus on LAG-3 and TIM-3 expression on immune cells, our group observed expression of LAG-3 and TIM-3 in tumour cells of melanoma metastases. We aim to investigate melanoma cell intrinsic LAG-3 and TIM-3 expression using various methods, correlate this data with clinical data and study the functional role of LAG-3 expression in melanoma cells using in vitro model systems.
Funding: Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project number 259332240 / RTG 2099 and a scientific grant from BMS
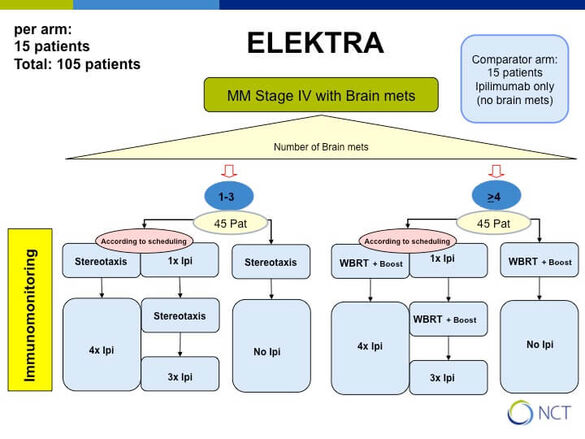
How does Ipilimumab and radiation interact in melanoma patients with brain metastases (ELEKTRA)
Ipilimumab is a CTLA-4 antibody which blocks downregulation of activated T cells and is successfully used to treat patients with metastasized melanoma. However, its activity in the brain is limited to small asymptomatic lesions and hence it is routinely combined with radiation of brain metastases. It is believed that the release of tumor antigens by radiation might enhance response to immune checkpoint blockers but not much is known on the mechanisms.
In cooperation with P. Beckhove, Regensburg we aim to detect differences in the peripheral blood between patients who receive either stereotactic (1-3 brain metastases) or whole brain radiotherapy (WBRT, > 3 brain metastases) depending on the treatment schedule either starting with radiation or with immunotherapy (see study design).
Primary endpoint: immunological and imaging response at 3 months after completion of radiation in the different treatment arms
Immunologic investigations include FACS analysis for T cell composition, IFN-gamma Elispot analysis of separated CD4 and CD8 T cells for reactivity against 5 major melanoma associated antigens and detection of tumor antigen specific Treg
Funding: Bristol Myers Squibb, Research Grant
Staff: I. Hülsmeyer, BTA
Relationship between TIL repertoire and treatment impact in human melanoma
Tumor infiltrating lymphocytes (TIL) play a crucial role in the therapeutic impact of immune checkpoint blockers, and these T-cell fractions can also be effectively used in the context of TIL therapy, which involves the harvest, ex vivo expansion and reinfusion of autologous TIL.
In the frame of the BMBF-Junior consortium TIL-REP we aim to investigate:
- Identification of biomarkers for clinical response through immunohistochemical analysis of TIL before and during treatment with immune checkpoint blockers
- Identification of tumor-reactive TILs and their use as biomarkers through TCR repertoire profiling of the TIL and PBMC compartments before and during treatment
- Enabling TIL therapy in our clinic through optimization of TIL isolation and expansion
These studies should not only help to identify biomarkers for response, but are crucial to find out if a combination of TIL infusion and treatment with immune checkpoint blockers might be useful. These data will be used to eventually plan a phase 1 trial combining TIL treatment with immune checkpoint blockers.
http://www.sys-med.de/de/nachwuchsforschung/juniorverbuende/til-rep/tp-4/
Funding: BMBF Research Grant
Staff: J. Roth, BTA; J. Winkler/C. Bender, rotating residents
STAT5 as resistance mechanism for interferon treatment in melanoma
STAT5 is a transcription factor involved in signal transduction pathways inducing anti-apoptotis and proliferation. From previous investigations in the fish melanoma model of Xiphophorus maculatus it is known that STAT5 plays a crucial role in cell survival and proliferation of melanoma cells (Morcinek et al, 2002; Wellbrock et al, 2005; Hassel et al, 2008).
Interferon-alpha is used adjuvant to treat patients with stage II/III melanoma. Besides the influence on the immune system e.g. by upregulation of MHC class I and II molecules and activation of macrophages interferons have a direct anti-oncogenic effect in vitro. Here, interferon receptors induce growth inhibition in melanoma cells via activation of STAT1. Mutation of STAT1 is known to cause melanoma resistance. In interferon resistant melanoma cell lines we found a high expression of STAT5 and a low expression of STAT1. Hence, this might be a hint for interferon resistance, but nothing is known if this plays a role in vivo.
The aim of this project is to evaluate STAT1,3 and 5 expression in melanoma metastasis by immunohistochemistry and evaluate activation of the transcription factors by the use of phophos-antibodies in immunofluorescence staining. Results will be correlate dot the clinical course.
Funding: Department funds
Staff: J. Roth, BTA; J. Winkler, rotating resident
Ex vivo melanoma tissue cultures to test treatment combinations and interactions
In the last years new treatment options emerged for patients with metastatic melanoma. These include targeted treatments with BRAF and MEK inhibitors as well as the immune checkpoint blockers Ipilimumab and the PD-1 antibodies. And there are many other antibodies and small molecules in pipeline tested in early clinical trials. Hence, there will be many possibilities to combine drugs or for sequential schedules, e.g. for the combination of targeted and immunotherapies.
In an ex vivo system where 300µm thick tissue slices from melanoma metastases will be taken into culture we aim to test substances on their effect on tumor cells, e.g. the expression of PD-L1.
Funding: Department funds
Staff: J. Roth, BTA; N. Lang
Group members
Bio.-Techn. Assistenten/-innen
-

Stefanie Friese
-

Oksana Hautzinger
-

Jasmin Richter
Wiss. Mitarbeiter/-innen
-

Timon Blindauer
-

PD Dr. med. Susanne Dugas-Breit
Fachärztin für Dermatologie und Venerologie
Dermatopathologie, Berufsdermatologie (ABD), Allergologie,
Fachkunde im Strahlenschutz (perkutane Röntgentherapie)Schwerpunkt
Dermato-Onkologie, PROMs
-

Devayani Machiraju
-
PD Dr. med. Robin Niklas Reschke
Haut- und Lymphknotensonographie (DEGUM)
Schwerpunkt
Dermato-Onkologie, Tumorimmunologie
Selected Publications
Hassel JC, Groesser L, Herschberger E, Weichert W, Hafner C (2015). RAS Mutations in Benign Epithelial Tumors Associated with BRAF Inhibitor Treatment of Melanoma. J Invest Dermatol. 135:636-9.
Hassel JC, Amann PM, Schadendorf D, Eichmüller SB, Nagler M, Bazhin AV (2013). Lecithin retinol acyltransferase as a potential prognostic marker for malignant melanoma. Exp Dermatol. 22:757-9
Hassel JC, Sucker A, Edler L, Kurzen H, Moll I, Stresemann C, Spieth K, Mauch C, Rass K, Dummer R, Schadendorf D (2010). MGMT gene promoter methylation correlates with tolerance of temozolomide treatment in melanoma but not with clinical outcome. Br J Cancer 103: 820-6
Hassel JC, Winnemöller D, Schartl M, Wellbrock C (2008). STAT5 contributes to anti-apoptosis in melanoma. Melanoma Res 18: 378-385
Wellbrock C, Weisser C, Hassel JC, Fischer P, Becker J, Vetter CS, Behrmann I, Kortylewski M, Heinrich PC, Schartl M (2005). STAT5 contributes to interferon resistance of melanoma cells. Curr Biol 15: 1629-1639
Morcinek JC, Weisser C, Geissinger E, Schartl M, Wellbrock C (2002). Activation of STAT5 triggers proliferation and contributes to anti-apoptotic signalling mediated by the oncogenic Xmrk kinase. Oncogene 21: 1668-1678