Research Area A: “Skin immune barrier functions”
The focus of area A is to define the role of molecules and microbes challenging the skin immune system from outside and mainly deals with innate immune reactions.
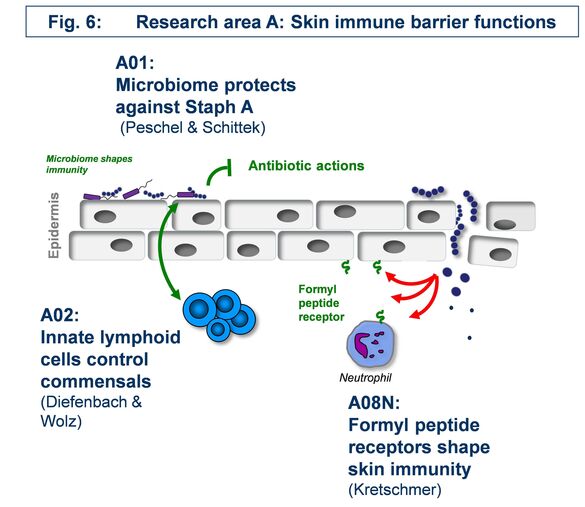
A01 - The skin microbiome in resistance against Staphylococcus aureus colonization
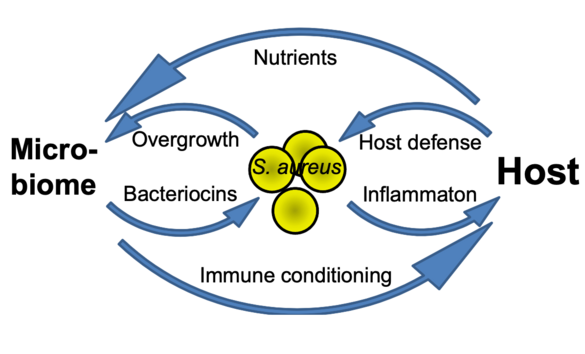
How beneficial, ‘commensal’ skin bacteria can protect against Staphylococcus aureus colonization and how such processes can be exploited for preventive and therapeutic avenues e.g. in atopic dermatitis remains to be elucidated. We found the tripartite interaction of S. aureus, commensals, and the host to depend either on direct interaction e.g. via the novel microbiome-derived antibiotic lugdunin or on influencing skin defense mechanisms. We propose a comprehensive research program on the mechanisms of microbiome-mediated pathogen exclusion and on microbiome-host cell communication as a basis for innovative interventions in microbiome-associated skin diseases.
Principal investigators
A02 - Innate immune-mediated control of skin epithelial cell renewal and staphylococcal commensalism
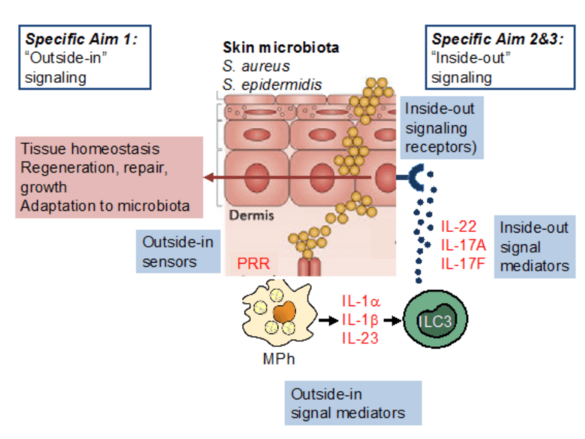
The skin is colonized by a complex microbiota, including staphylococci. Our hypothesis is that staphylococci and, in particular S. aureus, provide essential signals to control the activity of mononuclear phagocytes and ILC3, thereby regulating epithelial function. We will interrogate S. aureus-derived factors and their regulatory machinery to understand why S. aureus is such a potent enhancer of skin ILC3 function, extend our analysis of the role of IL-22 on skin epithelial stem cells in the context of staphylococcal colonization, and analyse how immune system components (i.e., IL-22, IL-17A, IL-17F) may impact bacterial gene expression.
Principal investigators
A08 - Consequences of formyl-peptide receptor activation to the skin
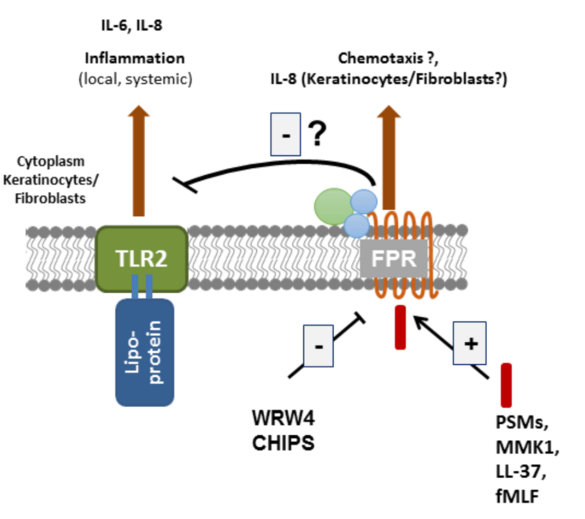
Leukocytes sense formylated peptides via formyl-peptide receptors (FPRs). Staphylococcal skin commensals and pathogens secrete phenol-soluble modulin (PSM) peptides, strong agonists of FPR2. FPR2 is important for the recruitment of leukocytes in early infections with S. aureus in mice. Although FPR2 is expressed also on human keratinocytes and skin fibroblasts, nearly nothing is known about the consequence of FPR activation in these cells. Our aim is to elucidate the role of FPRs and FPR-activating ligands in the inflammatory responses and in signal transduction in primary keratinocytes and skin fibroblasts. We will use cell culture and mouse models of skin wound healing, infection and systemic sclerosis to assess the potential of FPRs as targets for the prevention or therapy of skin diseases.