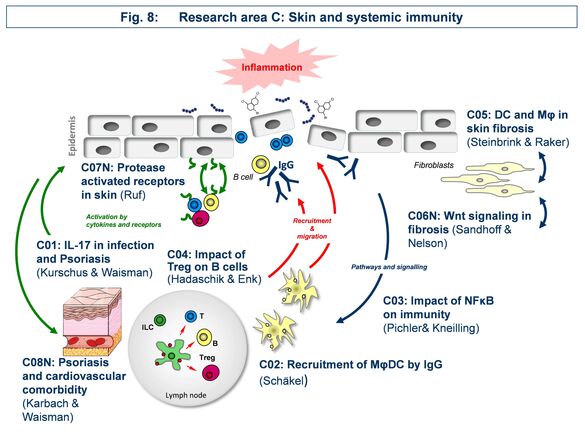
Research Area C: “Skin and systemic immunity”
Area C focusses on the effect of skin immune reactions for triggering autoinflammatory diseases such as psoriasis, autoimmune blistering disease, or scleroderma.
C01 - Targets and mediators of IL-17 in mouse models of psoriasis and skin infection
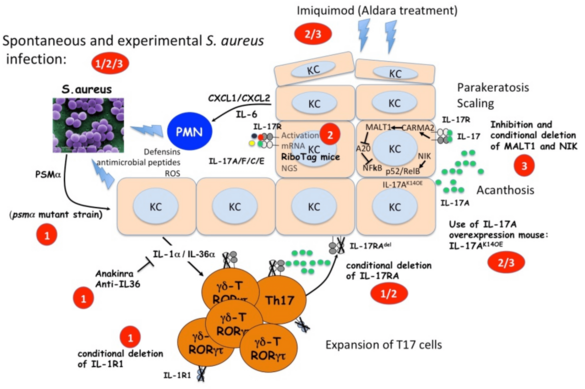
The IL-17 response plays a major role in the defense against S. aureus but also in the pathogenesis of psoriasis. As we are interested in the specific mechanism of IL-17 induction and its downstream pathways we will (i) investigate how T cells expressing IL-17 are induced by S. aureus infections; (ii) analyze the keratinocyte gene expression response to IL-17 in different models of psoriasis as well as in S. aureus infection; (iii) investigate the role of the MALT1-NFκB pathway and the NIK non-canonical NFκB pathway in the keratinocyte response to IL-17A.
Principal investigators
C03 - Impact of noncanonical NF-κB signaling during hapten-induced skin inflammation
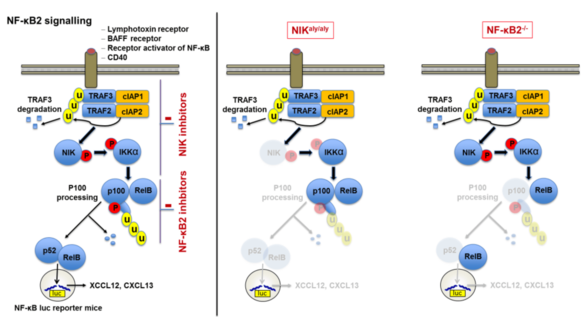
Our studies revealed that noncanonical NF-κB signaling is essential for adaptive immunity as mice deficient in NF-κB2 signaling were unable to develop progressive chronic contact hypersensitivity reactions. Thus, we aim to uncover how noncanonical NF-kB signaling modulates acute and chronic T cell driven inflammatory processes by (i) identifying the essential cellular players employing cell-type specific conditional NF-κB2-/- and NIKaly/aly mice in vivo, (ii) investigating cell specific impairments in activation, differentiation and survival in vitro, (iii) analyzing differences in T cell activation and leukocyte homing patterns in vivo and (iv) uncovering the efficacy of specific NF-κB2 inhibition in vitro and in vivo.
Principal investigators
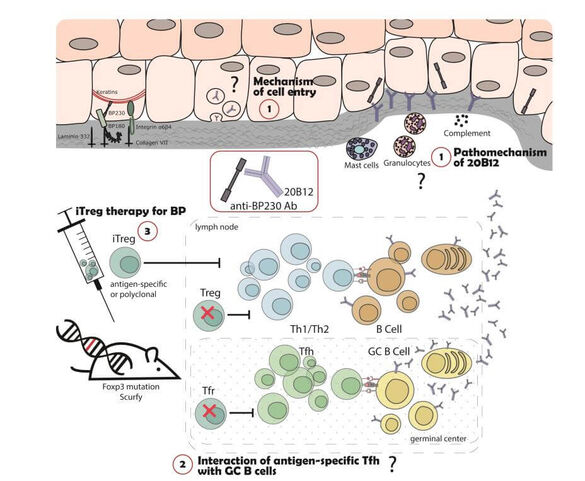
C04 - Role of regulatory T cells in B cell-mediated autoimmune skin diseases
Autoimmune bullous dermatoses (AIBD) are severe autoantibody-mediated skin diseases. The pathogenic relevance of autoreactive CD4+ T cells for the induction of autoantibody production is unclear. We have previously shown that Treg-deficient Scurfy mice spontaneously develop autoantibodies against different AIBD autoantigens. Now we aim to further investigate this aspect of AIBD development in the Scurfy model: Our project will address, (i) which different types of AIBD develop in Scurfy mice, (ii) dissect the pathophysiologic mechanisms of the pathogenic autoantibodies and (iii) focus on targeting antigen-specific CD4+ T cells as therapeutic strategy in AIBD.
Principal investigators
C05 - Scleroderma: role of macrophages and dendritic cells in tissue fibrosis
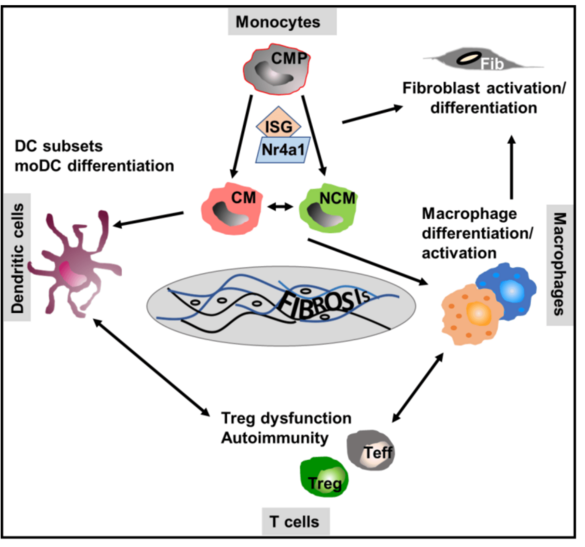
The function of monocyte/macrophage differentiation and of dendritic cells in the pathogenesis of scleroderma (Scl), the skin manifestation of systemic sclerosis with extensive fibrosis, is not clear. We will alter the frequency, polarization and migration of myeloid/dendritic cells to test Scl development and the underlying immune mechanisms in chemically induced and spontaneous models of the disease. In addition, we will compare these data with that of monocytes/myeloid cells in the blood and the skin of patients suffering from systemic sclerosis to identify key (innate) cellular myeloid drivers and dominant molecular mechanisms of Scl.
Principal investigators
C06 - Wnt-associated Dkk3 in inflammatory and fibrosing skin disease
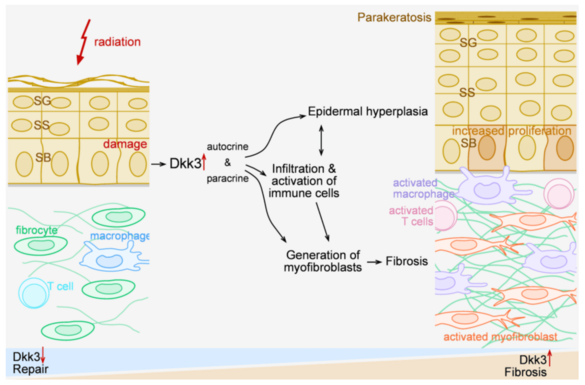
Radiation dermatitis can initiate a chronic inflammatory/fibrosing process. We found that Dickkopf-3 (Dkk3), a Wnt-related protein linked to immune regulation, promotes chronic fibrosing inflammation in mouse models of radiation damage. Dkk3 knockouts do not develop epidermal hyperplasia, or chronic dermal fibroblast/inflammatory activation. The proposal seeks to (i) identify the relevant cellular source of Dkk3 in radiation damaged skin using reporter mice and targeted Dkk3 knockouts, (ii) characterize Dkk3 effects on local macrophages and T cells, (iii) dissect the impact of Dkk-related signalling pathways using human skin models, and (iv) connect radiation-induced epigenetic modifications to expression of Dkk3 and chronic inflammatory fibrosis.
Principal investigators
C07 - PAR2 signaling in cutaneous inflammation
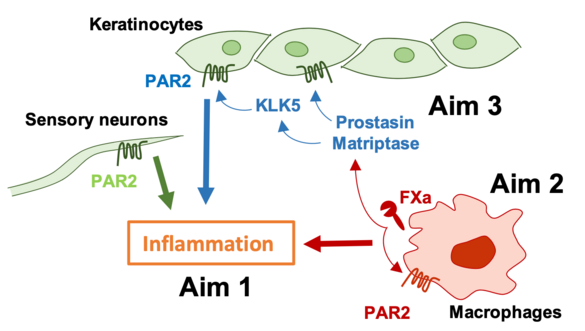
Proteolytic signaling through the G protein-coupled protease activated receptor (PAR) 2 promotes contact hypersensitivity (CHS) reactions, but relevant cell types and participating proteases are poorly defined. We will employ cell type-specific deletion of PAR2 in keratinocytes, sensory neurons and macrophages as well as novel mouse models with altered PAR2 signaling by specific proteases to delineate the contributions of PAR2 to skin inflammation. These experiments will reveal how coagulation and other proteases contribute to initiation and amplification of CHS responses and other inflammatory skin disorders.
Principal investigators
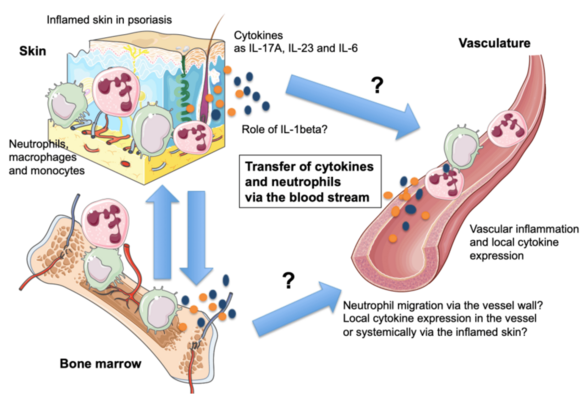
C08 - Resolving the mechanisms of the skin-vasculature-crosstalk in psoriasis-associated vascular dysfunction
Psoriasis is a common skin condition manifested by high levels of the pro-inflammatory cytokine IL-17A. Ectopic expression of IL-17A by keratinocytes leads to the development of early skin inflammation followed by vascular inflammation and dysfunction as in patients with severe psoriasis. To investigate the link between psoriasis and vascular inflammation, we will 1.) study whether inflammatory cells can migrate from the skin to the cardiovascular system. 2.) Study whether ablation of neutrophils that invade the skin in psoriasis leads to diminished vascular disease. We will generate mice with specific deletion of CD18 in neutrophils, in order to prevent their extravasation via the vessel wall, to study the role of these cells in psoriasis-like skin disease and the associated vascular inflammation. 3) We plan to compare cytokine profiles in aorta, skin and blood of psoriatic mice to control animals and with skin and blood samples of psoriasis patients. 4) We will analyze if vascular inflammation in psoriasis can be inhibited by blocking IL-1 signaling.