Research Projects
Dynamic rearrangements of the actin cytoskeleton in T cells
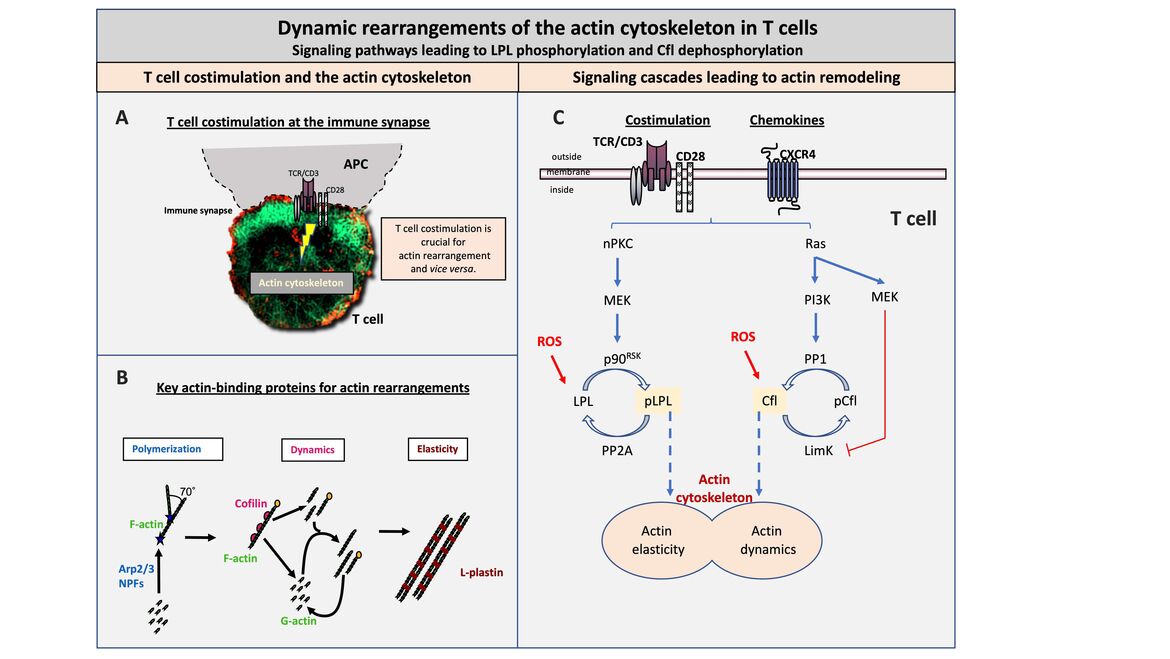
T cell costimulation at the immune synapse
T cells are highly mobile cells that patrol the body in search of the appropriate antigen as part of the immune surveillance. When the cognate antigen is found on antigen-presenting cells (APCs), a transient, but stable contact zone is formed between T cells and APCs that functionally resembles a neuronal synapse. Therefore, this contact zone was named immune synapse. The immune synapse is essential for communication between T cells and APCs and for target cell killing.
The formation of the immune synapse is dependent on T cell costimulation and a remodeling of the actin cytoskeleton. Interestingly, the actin cytoskeleton is in turn also essential for T cell costimulation. Thus, the actin cytoskeleton represents a central switch point for the adaptive immune response. Genetic defects in the regulation of the actin cytoskeleton are therefore inevitably associated with immunodeficiency (e.g. the Wiskott Aldrich Syndrome).
Key actin-binding proteins for actin rearrangement
The actin cytoskeleton is modular in structure with globular actin (G-actin) as the basic unit. G-actin is polymerized to filamentous actin (F-actin) by so-called nucleation promoting factors (NPF) and the AR2/3 complex. Single actin filaments can be docked at an angle of 70° to already existing F-actin (left, Polymerization). F-actin can be either severed or depolymerized by cofilin in a very dynamic process. Depending on which process predominates, cofilin causes either depolymerization or enhanced polymerization (middle, Dynamics). Actin elasticity can be induced by crosslinking or bundling of F-actin. This stabilization of the actin backbone is controlled by special crosslinking/bundling proteins, such as L-plastin (LPL) (right, Elasticity).
Signaling cascades leading to actin remodeling
The activity of the actin dynamics inducing protein cofilin and the actin-bundling protein LPL are regulated downstream of costimulation or chemokine triggering by reversible phosphorylation. LPL activity is enhanced by phosphorylation at serine 5 (Ser5) mediated by an nPKC-MEK-p90RSK signaling pathway. The serine-threonine phosphatase PP2A dephosphorylates pLPL at Ser5 leading to a reduction in activity. Cofilin, in turn, is inactive in the Ser3 phosphorylated state and is dephosphorylated and thus activated by a Ras-PI3K-PP1 cascade. Simultaneously, the cofilin kinase LimK is inactivated via Ras-MEK, resulting in stable dephosphorylation of cofilin. The activity of both proteins can be modulated by reactive oxygen species (ROS) and Antioxidants as factors of the cell microenvironment.
Figure legend
(A) T cell costimulation at the immune synapse (B) Key actin-binding proteins for actin rearrangement (C) Signaling cascades leading to actin remodeling
Influencing skin inflammation by targeting T cell co-stimulation and leukocyte actin dynamics
Inflammatory skin diseases caused by dysregulated immune responses are a common problem occurring in humans of all ages and having a major impact on the quality of life of the patients.
For the establishment of an immune reaction, cell-cell interactions and a dynamic reorganization of the actin cytoskeleton in immune cells are essential.
To gain deeper insight into these processes, we focus on influencing actin dynamics and on interfering with the interaction between antigen-presenting keratinocytes and T cells during skin diseases. With the help of human as well as murine systems we want to establish novel immunomodulatory strategies helping to generate the basis for future therapies.
This project is funded by the SFB Transregio 156 “The Skin as Sensor and Effector Organ Orchestrating Local and Systemic Immune Responses”.
Figure legend
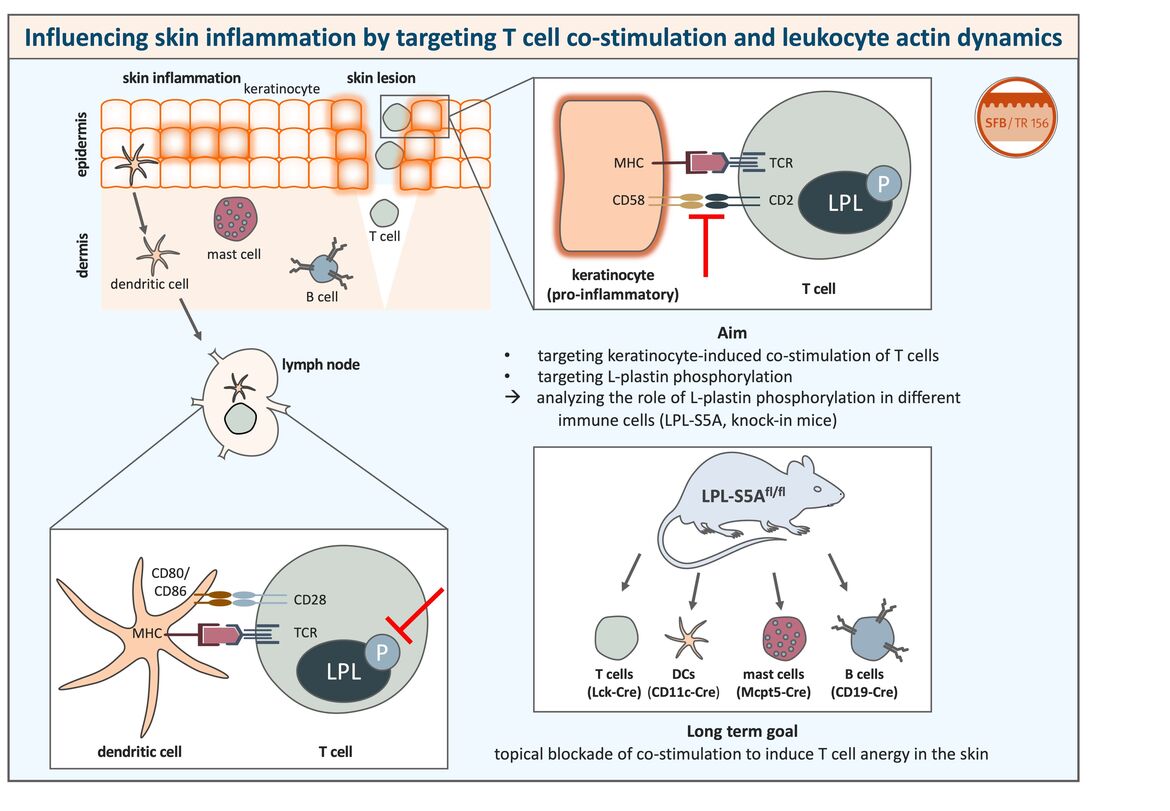
(Left) The skin contains numerous immune cells that are defending the host against pathogens. However, a dysregulation of the immune reaction can lead to inflammatory skin diseases. Skin-resident dendritic cells can take up antigen and migrate to draining lymph nodes where they interact with naïve T cells to induce an immune response. Peptides bound to MHC molecules presented by dendritic cells are recognized by their cognate T cell receptor (TCR) on the T cell surface. For proper T cell activation, binding of T cell co-stimulatory receptors like CD28 to their ligands on the antigen-presenting cell (e.g. CD80/CD86) and phosphorylation of the actin-bundling protein L-plastin are necessary as a second signal.
(Right) We could show that, under pro-inflammatory conditions, keratinocytes can also act as antigen-presenting cells and activate even naïve T cells that are for example present in skin lesions. Co-stimulatory receptor ligands on keratinocytes include for example CD58, but they do not express ligands for CD28, CD80 and CD86, which are typically expressed on professional antigen-presenting cells.
The aim of this project is to target keratinocyte-induced co-stimulation of T cells and L-plastin phosphorylation in T cells in order to block T cell co-stimulation in the skin. A blockade of L-plastin phosphorylation would probably also influence other immune cells besides T cells, since L-plastin is a leukocyte-specific protein. To analyze the function of L-plastin activation in other immune cells as well, we make use of genetically modified mice expressing a non-phosphorylatable form of L-plastin cell type-specifically.
The topical blockade of T cell co-stimulation in order to induce T cell anergy in the skin could improve the treatment of inflammatory skin diseases such as psoriasis or contact allergy in which undesired T cell activation plays a major role.
Plant extracts and lead substances – novel prospects for treating inflammatory diseases?
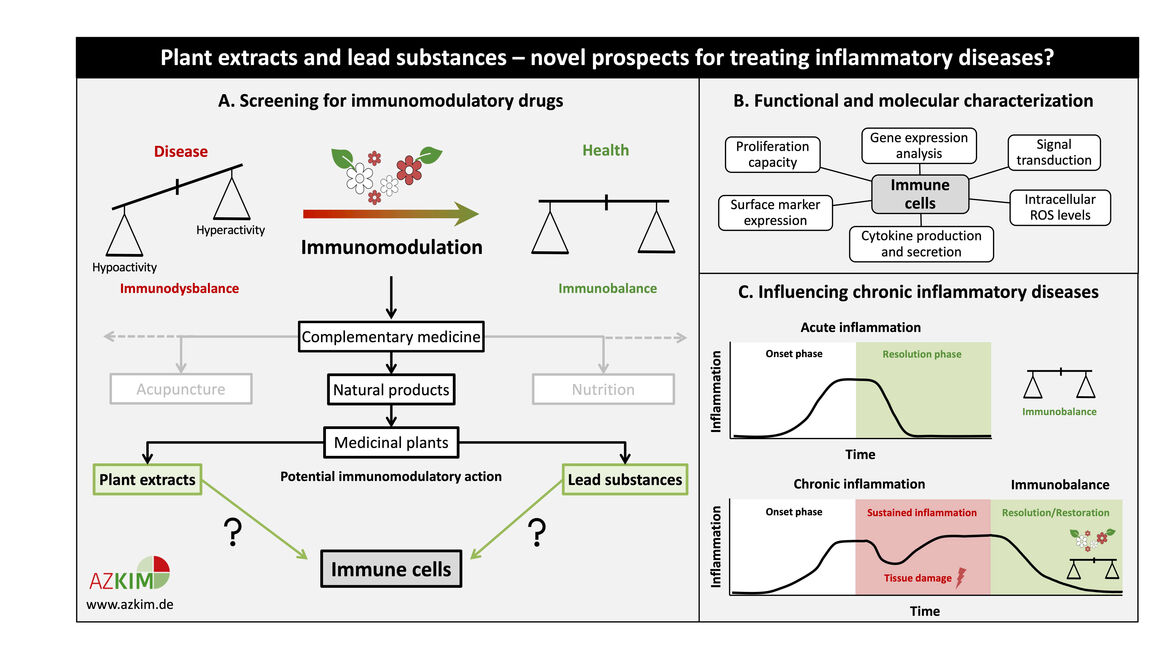
Numerous disease states are characterized by a skewed immune response. Immunomodulatory substances can either stimulate or suppress the innate and/or adaptive immune system in order to restore immune homeostasis. Since the prevailing administration of most immunomodulators in clinical use exhibits severe side effects, medicinal plants and their active components constitute a promising complement to conventional therapies.
This project will elucidate the immunomodulatory potential of plant extracts and active ingredients through functional and molecular characterization. Selected non-toxic drug concentrations are tested for their effect on leukocyte proliferation, activation-induced changes in surface marker expression, etc. Following screening, promising candidates will undergo investigation of the underlying molecular mechanism.
Our studies will enable a critical evaluation of the effects of complementary medicine on the immune system based on consolidated experimental data, thereby improving conventional treatment strategies for immune-related diseases like chronic inflammation. In this context, plant-based drugs or food supplements might reinstate resolution of inflammation and help to restore immune balance. Sulphoraphane (from cruciferous vegetables) and Piperlongumine (from Piper longum) are two examples for lead substances which showed an anti-inflammatory effect on primary human T cells in our previous studies. These drugs might offer novel options for the treatment of inflammatory diseases like rheumatoid arthritis.
Figure legend
(A) Schematic representation of the screening for potential plant-based immunomodulators. Promising plant extracts and/or lead substances may be used to restore immune homeostasis in those diseases that are characterized by a dysbalanced immune system. (B) Overview of assays performed for functional and molecular characterization of potential plant-based immunomodulators. (C) Possible clinical application of plant extracts and lead substances for the treatment of chronic inflammatory diseases. Sustained inflammation can be resolved using appropriate anti-inflammatory candidates. Vice versa, also immune stimulatory candidates will be selected for further application.
Empowering CAR T cells against ROS-mediated immunosuppression in solid tumors
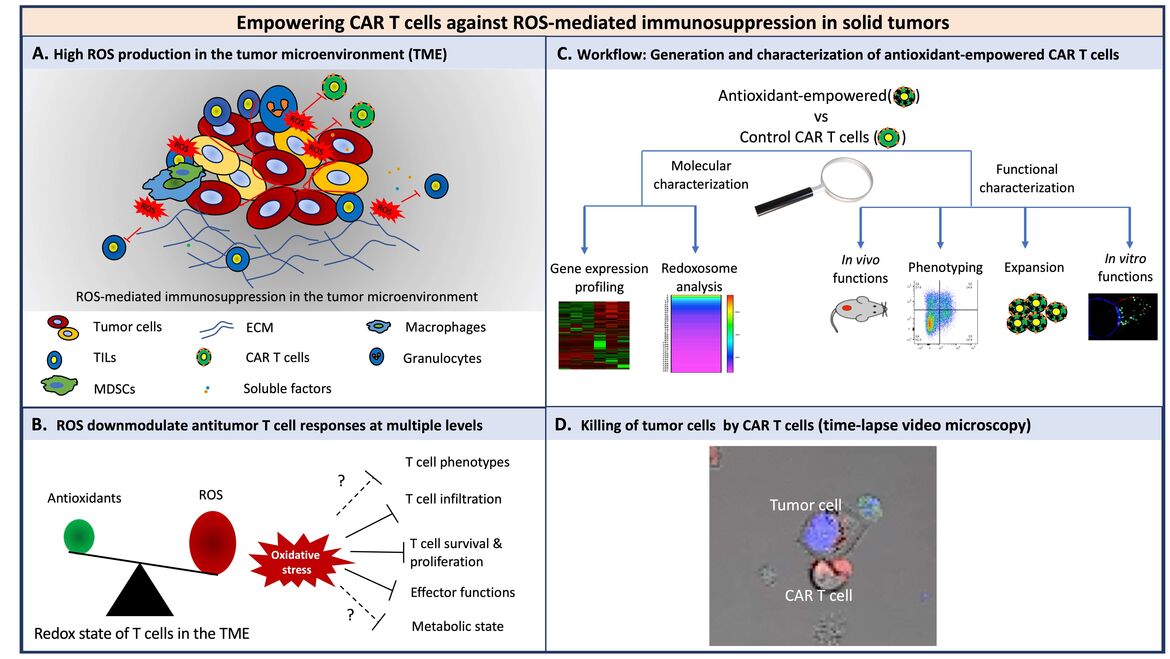
Under pro-oxidative conditions, T cells are hypo-responsive or even undergo programmed cell death. Accordingly, the pro-oxidative micromilieu of solid tumors provides strong challenges to T cells through dampening T cell responses in several ways by induction of protein cysteine oxidation. In this context, the efficacy of CAR therapy against solid tumors is limited potentially due to the immunosuppressive microenvironment, although it has shown great promises against lymphomas and leukemias. Thus, we investigate the components of the micromilieu of solid tumors, particularly the redox micromilieu, and their influence on T cells at the molecular and cellular level. We engineer CAR T cells overexpressing genes which make them resistant to such a micromilieu. Currently, we are investigating the activity, longevity and effector functions of empowered CAR T cells in vitro as well as in vivo. Altogether, our findings will decipher whether engineered T cells equipped with a higher antioxidative capacity could improve T cell therapies.
Figure legend
(A) Various cells in the TME contribute to the pro-oxidative state. (B) ROS downmodulate antitumor T cell responses at multiple levels. Solid lines indicate downmodulation-, dashed lines and question marks indicate “potential” downmodulation of T cell functions. (C) Workflow to characterize the antioxidant empowered CAR T cells for more efficient therapies against solid tumors. (D) A representative time-lapse video microscopy of CAR T cells and tumor cells. Prior to the coculture, cytolytic granules of T cells were prestained with lysotracker red (red) and tumor cells were prestained with DAPI (nuclei, blue) and caspase3/7 sensor, green).
Neutrophils as mediators of chronic inflammation
Heterogeneity, plasticity and functionality of human neutrophils
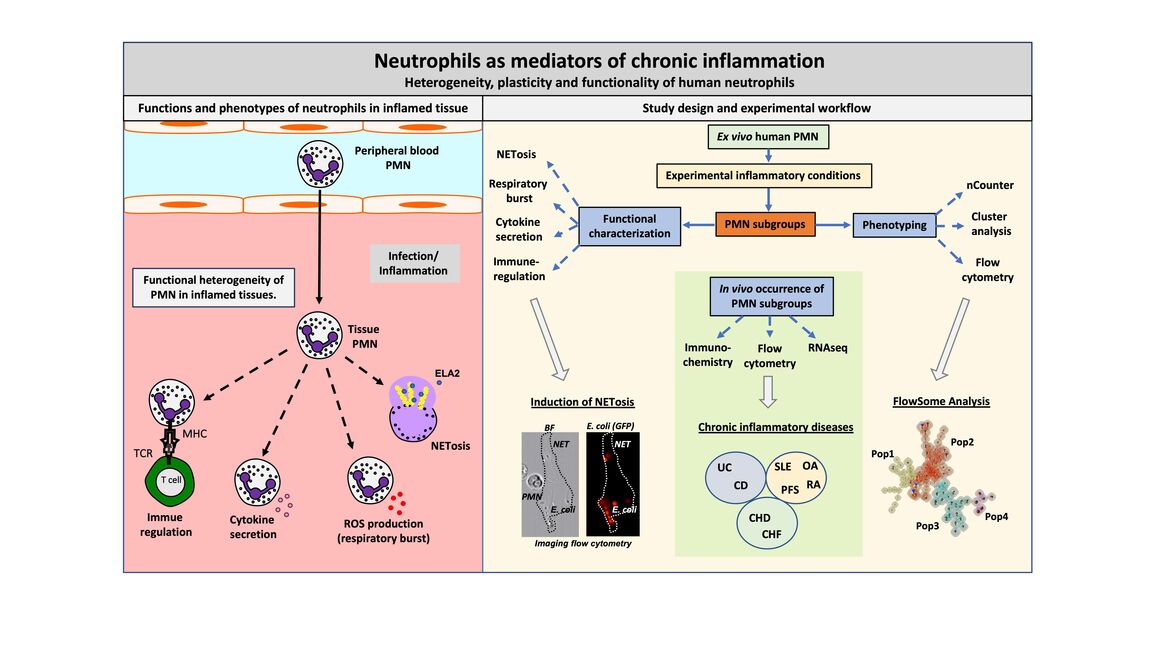
Neutrophilic granulocytes (PMN) are among the first cells to fight bacterial infections. Although it was long believed that PMN are a homogenous cell population there is now consensus in science that many PMN subgroups, differentiation and activation states exist - especially in inflamed tissues. These subgroups have different functional properties. We analyze the development of human PMN activation states and subgroups under inflammatory conditions in vitro and in vivo. Thereby, we have identified a novel PMN subgroup induced by pro-inflammatory cytokines, that expresses the chemokine-receptor CCR5 and the TNF-receptor TNFR2, named CCR5+ cytokine-induced PMN (CCR5+ cPMN). This subgroup has a pro-NETotic phenotype and occurs in chronic inflammatory diseases, e.g. ulcerative colitis. The relevance of this and other cytokine induced subgroups in health and disease are currently investigated
Figure legend
(Left) Functions and phenotypes of neutrophils in inflamed tissue
PMN are the most abundant cell type in the human peripheral blood. They emigrate into infected or inflamed tissues, where a complex interplay between the tissue micro-environment and PMN occurs. Tissue PMN modify the micro-environment by NETosis, ROS production and cytokine secretion. Moreover, they can directly interact with T-cells and, thereby, modulate adaptive immune responses. It is recognized that functional and phenotypic heterogeneity exist, but it is not fully understood to which part PMN subgroups contribute to the different functional outcomes. Moreover, it is unknown which factors induce PMN heterogeneity and whether PMN subgroups are temporally related activation states or divergent, independent subpopulations. This question is addressed in this project.
(Right) Study design and experimental workflow
To analyze such diversification processes, human PMN are isolated from the peripheral blood of healthy donors. These resting PMN are activated by cytokines to mimic inflammatory conditions and, thus, to induce PMN heterogeneity. The PMN subgroups are phenotyped by gene expression profiling (nCounter), cluster analysis and flow cytometry/ FlowSome giving rise to 4 PMN subgroups. Functional characterizations are now performed for activated pan-PMN as well as for PMN subgroups. These include the quantification of NETosis, respiratory burst, cytokine secretion and immune regulation by state-of-the-art imaging and cell biology related methods. To scrutinize whether the characterized PMN subgroups occur in vivo, the peripheral blood and tissue of patients suffering from chronic inflammatory diseases are analyzed.
(Abbreviations: PMN= Neutrophilic granulocytes; NET=Neutrophil extracellular trap; ELA2=Neutrophil elastase; ROS=Reactive oxygen species; GFP=green fluorescent protein; TCR=T-cell receptor; MHC=Major histocompatibility complex; UC=Ulcerative colitis; CD=Crohn´s disease; SLE=Systemic lupus erythematosus; OA=Osteoarthritis; RA=Rheumatoid arthritis; PFS=Periodic fever syndromes; CHD=Coronary heart disease; CHF=Chronic heart failure)
Microscopy: platform and method development
Figure legend
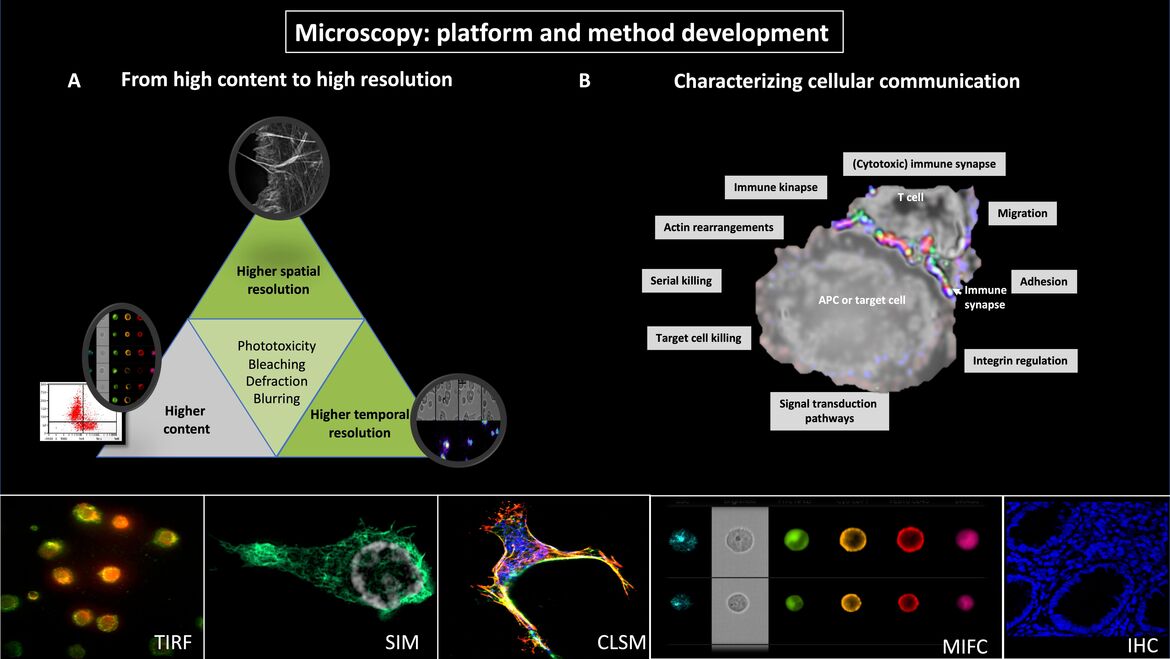
(A) From high content to high resolution
Fluorescence microscopy is essential for the study of cellular and molecular mechanisms in leukocytes. However, it is not possible to simultaneously perform high-content and high spatial and temporal resolution. Instead, the microscopic system and the corresponding imaging method need to be optimized for one specific question. Our methodological and microscopical platform includes multispectral imaging flow cytometry (MIFC), confocal laserscan microscopy (CLSM), total internal reflection microscopy (TIRF), wide-field and confocal life-cell imaging and the super-resolution microscopy technique structured illumination microscopy (3D-SIM). These allow the sequential collection of high-content data (MIFC), high or super-resolved images (3D-SIM or TIRF) and fast processes as calcium flux (CLSM).
(B) Characterizing cellular communication
We developed methods for capturing cell adhesion and migration as well as for imaging cellular communication of leukocytes. MIFC allows to acquire images of thousands of cells in a relatively short time period and to analyze morphometric data in semi-automated batch procedures. Biological dynamics can be analyzed in heterogenous cell populations and MIFC-based gating on the population of interest. These analyses are then complemented by time-lapse videomicroscopy (TLV) and 3D-SIM with very high spatial resolution so that approaches with different strengths can be logically combined.