Center for Translational Biomedical Iron Research (CeTBI, Zentrum für translationale und biomedizinische Eisenforschung)
Head of Research
Prof. Dr. phil. nat. Martina Muckenthaler
Head of Molecular Medicine, MMPU Group Leader
Basic research
Mechanisms underlying adequate iron supplies (MMPU project)
Background
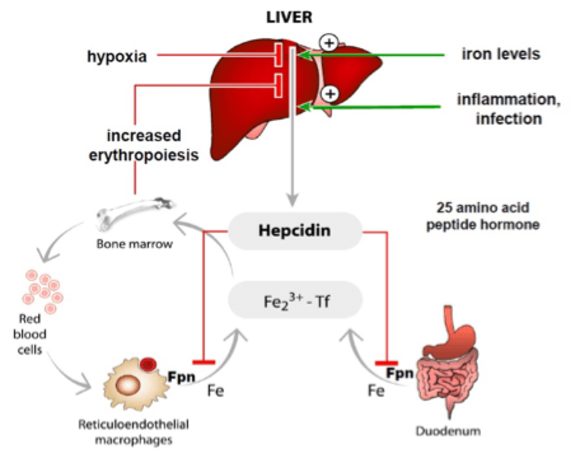
Iron homeostasis must be maintained to assure that tissues and cells receive sufficient amounts of iron, while iron overload is prevented. Iron levels are maintained within the physiological range by a hormonal system, the hepcidin/ferroportin regulatory axis. The peptide hormone hepcidin is produced by the liver and controls iron fluxes by degrading the iron exporter ferroportin.
High hepcidin levels, that arise as a consequence of iron overload or inflammation prevent excess iron absorption from the diet and iron release from macrophages that recycle iron from aging red blood cells. Low hepcidin levels are observed when iron supplies are low and insufficient iron is available for red blood cell synthesis. As a consequence, increased amounts of iron are taken up from the diet.
Dr. Sandro Altamura (senior post-doc), Silvia Colucci (PhD student):
"An important aim of our research is to understand signals and pathways that are responsible for the synthesis of the hormone hepcidin. We ask the question how iron availability is measured in the liver and how adequate hepcidin levels are produced."
Dr. Oriana Marques (post-doc), Laura Zechner (master student):
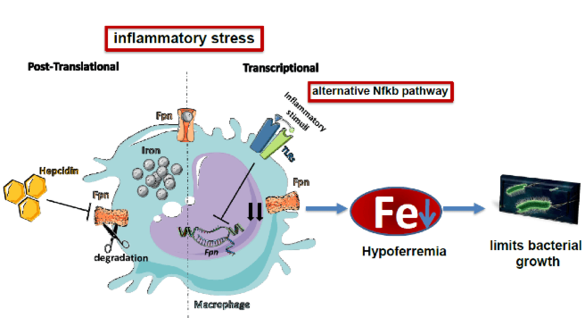
"During an infection the amount of iron is reduced in the blood (hypoferremia). This is a critical innate immune response to reduce growth of bacteria that require iron as a nutrient. We want to understand the signals and pathways that reduce iron availability. Of special interest is the question how iron export is reduced via ferroportin as a consequence of inflammation."
National/international cooperation
This project is integrated into the German Center for Lung Research (DZL) and the Translational Lung Research Center Heidelberg (TLRC)
Selected publications:
Thielmann CM, Costa da Silva M, Muley T, Meister M, Herpel E, Muckenthaler MU. Iron accumulation in tumor-associated macrophages marks an improved overall survival in patients with lung adenocarcinoma. Sci Rep. 2019;9(1):11326. doi:10.1038/s41598-019-47833-x
Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell 2017, Jan 26; 168(3):344-361. doi: 10.1016/j.cell.2016.12.034.
Costa da Silva M, Breckwoldt MO, Vinchi F, Correia MP, Stojanovic A, Thielmann CM, Meister M, Muley T, Warth A, Platten M, Hentze MW, Cerwenka A, Muckenthaler MU. Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages Frontiers in Immunology, 8. 2017. doi:10.3389/FIMMU.2017.01479
Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, Cerwenka A, Tolosano E, Muckenthaler MU. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 2016, Jan 28; 127(4):473-86. doi: 10.1182/blood-2015-08-663245.
Red blood cells, heme, iron, macrophages & cancer therapie (MMPU project)
Background
Tumor-associated macrophages (TAMs) are pro-tumoural cells that facilitate angiogenesis and vessel leakiness, conduct extra cellular and stromal matrix remodeling, and modulate both the innate and adaptive immune responses in favour of malignant progression and metastases. Therefore, the reprogramming of TAMs in the TME towards an inflammatory anti-cancer phenotype is a promising strategy to improve current anti-tumor therapies. Besides contributing to immune responses, macrophages recycle iron from red blood cells (RBCs). They engulf senescent RBCs and catabolize heme via the activity of heme oxygenases (HO-1 and HO-2) (1). Recently, we discovered that anti-inflammatory macrophages shift toward the pro-inflammatory state after exposure to heme or iron (2). These findings interconnect the dual functions of macrophages in iron handling and inflammation. In the context of hemolysis, e.g. in sickle cell disease, the reprogramming of macrophages in the liver as a consequence of heme exposure contributes to the generation of fibrosis in this tissue.
Hemolysis of RBCs also occurs in the tumor microenvironment. In the hemorrhagic areas of both human and mouse lung tumors (e.g. in NSCLC), red blood cells extravasate from vessels. The inflammatory environment triggers their damage and phagocytosis by TAMs. As a consequence, TAMs accumulate high amounts of iron and turn into iron-loaded TAMS (iTAMs). The occurrence of iTAMs correlates with reduced tumor size (3) and improved overall survival (4) of patients with non-small cell lung cancer (NSCLC). Ex vivo experiments established that TAMs exposed to hemolytic red blood cells (RBCs) were converted into pro-inflammatory macrophages capable of directly killing tumor cells. This anti-tumor effect can also be elicited via iron oxide nanoparticles in co-cultures and in vivo tumor models (3,5). These results identify hemolytic RBCs and iron as novel players in the TME that repolarize TAMs to exert direct anti-tumor effector function.
Natalie Horvath (PhD student):
"We aim to understand how red blood cells, heme and iron reprogram macrophages to an inflammtory phenotype and therapeutically use this potential to elicit anti-tumour activities. Furthermore, we want to assess the role of iron in macrophages in diverse disease settings."
National/international cooperation
This project is integrated into the German Center for Lung Research (DZL) and the Translational Lung Research Center Heidelberg (TLRC)
Selected publications:
Thielmann CM, Costa da Silva M, Muley T, Meister M, Herpel E, Muckenthaler MU. Iron accumulation in tumor-associated macrophages marks an improved overall survival in patients with lung adenocarcinoma. Sci Rep. 2019;9(1):11326. doi:10.1038/s41598-019-47833-x
Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell 2017, Jan 26; 168(3):344-361. doi: 10.1016/j.cell.2016.12.034.
Costa da Silva M, Breckwoldt MO, Vinchi F, Correia MP, Stojanovic A, Thielmann CM, Meister M, Muley T, Warth A, Platten M, Hentze MW, Cerwenka A, Muckenthaler MU. Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages Frontiers in Immunology, 8. 2017. doi:10.3389/FIMMU.2017.01479
Vinchi F, Costa da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, Cerwenka A, Tolosano E, Muckenthaler MU. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 2016, Jan 28; 127(4):473-86. doi: 10.1182/blood-2015-08-663245.
Iron and diabetic late complications
Background
Free excess iron is toxic as it reacts with oxygen to generate reactive oxygen species (ROS) which trigger cell damage. We showed that in patients with diabetes and in corresponding disease models iron homeostasis is unbalanced and that elevated iron levels in plasma and liver are considered a novel late diabetic complication.
Sandro Altamura (senior post-doc), Ruiyue Qiu (PhD student); Anand Ruban Agarvas (PhD student):
- How do iron-induced reactive metabolites contribute to the misregulation of the hepcidin/ferroportin regulatory system?
- How do elevated iron levels contribute to the late complications frequently observed in patients with diabetes?
National/international cooperation
This project is well integrated into the collaborative research network CRC 1118 (DFG).
Selected publications:
Altamura S, Kopf S, Schmidt J, Müdder K, da Silva AR, Nawroth P, Muckenthaler MU. Uncoupled iron homeostasis in type 2 diabetes mellitus. J Mol Med. 2017;95(12):1387–98. doi:10.1007/s00109-017-1596-3
Vinchi F, Porto G, Simmelbauer A, Altamura S, Passos ST, Garbowski M, Silva AMN, Spaich S, Seide SE, Sparla R, Hentze MW, Muckenthaler MU. 1.Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2020 Jul 21;41(28):2681-2695. doi: 10.1093/eurheartj/ehz112.
Groener JB, Oikonomou D, Cheko R, Kender Z, Zemva J, Kihm L, Muckenthaler M, Peters V, Fleming T, Kopf S, Nawroth PP. Methylglyoxal and Advanced Glycation End Products in Patients with Diabetes - What We Know so Far and the Missing Links. Exp Clin Endocrinol Diabetes. 2019;127(8):497–504. doi:10.1055/s-0043-106443
Senyilmaz-Tiebe D, Pfaff DH, Virtue S, Schwarz KV, Fleming T, Altamura S, Muckenthaler MU, Okun JG, Vidal-Puig A, Nawroth P, Teleman AA. Dietary stearic acid regulates mitochondria in vivo in humans. Nat Commun. 2018;9(1):3129. doi:10.1038/s41467-018-05614-6
Wiedenmann T, Dietrich N, Fleming T, Altamura S, Deelman LE, Henning RH, Muckenthaler MU, Nawroth PP, Hammes H-P, Wagner AH, Hecker M. Modulation of glutathione peroxidase activity by age-dependent carbonylation in glomeruli of diabetic mice. J Diabetes Complicat. 2018;32(2):130–8. doi: 10.1016/j.jdiacomp.2017.11.007
Brings S, Fleming T, Freichel M, Muckenthaler MU, Herzig S, Nawroth PP. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int J Mol Sci. 2017;18(5). doi:10.3390/ijms18050984
Altamura S, Kopf S, Schmidt J, Müdder K, da Silva AR, Nawroth P, Muckenthaler MU. Uncoupled iron homeostasis in type 2 diabetes mellitus. J Mol Med. 2017;95(12):1387–98. doi:10.1007/s00109-017-1596-3
Hidmark A, Fleming T, Vittas S, Mendler M, Deshpande D, Groener JB, Müller BP, Reeh PW, Sauer SK, Pham M, Muckenthaler MU, Bendszus M, Nawroth PP. A new paradigm to understand and treat diabetic neuropathy. Exp Clin Endocrinol Diabetes. 2014;122(4):201–7. doi:10.1055/s-0034-1367023
Iron and heart/vessels (MMPU project)
Background
While some degree of atherosclerosis is an almost inevitable consequence of ageing, food preferences, lack of exercise and other aspects of the lifestyle in many countries, it has propelled atherosclerosis into being a major health burden for billions of people. It has long been suspected that iron may contribute to and aggravate the development of atherosclerosis, but data have been conflicting and the contribution of iron is debated controversially. We recently provided clear evidence for the proatherogenic effect of iron overload. In addition, we demonstrated that dietary or pharmacological iron depletion results in a strong reduction of atherosclerotic lesions in mice and positively affects multiple clinical parameters of atherosclerosis in patients suffering from genetic iron overload. More recently we focused our attention on iron balance in the heart. On the one hand cardiac iron overload is observed in patients with hereditary hemochromatosis or thalassemia, where it causes heart failure. On the other hand up to 50% of patients with HF show systemic iron deficiency contributing to symptoms and poor prognosis. Since 2012 guidelines of the ESC recommend iron supplementation in HF patients – with unclear knowledge how this will affect the heart.
Christina Mertens (post-doc), Sandro Altamura (senior post-doc), Stefanie Martinache (technical Assistant):
- How are iron levels controlled in the heart?
- How does iron availability contribute to the outcome of heart failure?
Selected publication
- Vinchi F, Porto G, Simmelbauer A, Altamura S, Passos ST, Garbowski M, Silva AMN, Spaich S, Seide SE, Sparla R, Hentze MW, Muckenthaler MU. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur Heart J. 2019. doi:10.1093/eurheartj/ehz112
Iron, hypoxia and high altitude
Background
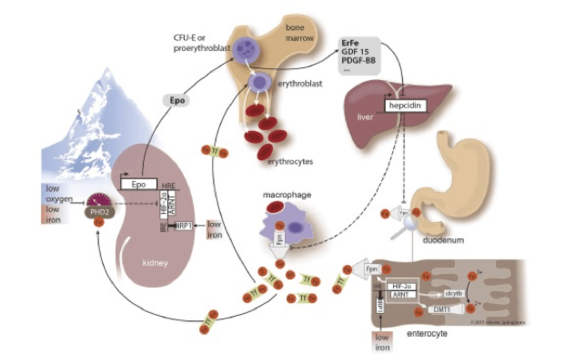
Both high altitude residents as well as sojourners to high altitude cope with decreasing inspiratory oxygen partial pressure by stimulating erythropoiesis. The increase in hemoglobin levels requires high amounts of additional iron supplied from the diet. We are interested in the mechanisms how iron metabolism adapts when living in a hypoxic environment.
Aims:
- We aim to understand mechanisms that link iron homeostasis with oxygen homeostasis.
- Furthermore, in a project initiated by the WHO we ask the question about the appropriate hemoglobin value in high altitude populations.
Selected publications
- Staub K, Haeusler M, Bender N, Morozova I, Eppenberger P, Panczak R, Zwahlen M, Schaer DJ, Maggiorini M, Ulrich S, Gassmann NN, Muckenthaler MU, Rühli F, Gassmann M. Hemoglobin concentration of young men at residential altitudes between 200 and 2000 m mirrors Switzerland's topography. Blood. 2020;135(13):1066–9. doi:10.1182/blood.2019004135
- Gassmann M, Mairbäurl H, Livshits L, Seide S, Hackbusch M, Malczyk M, Kraut S, Gassmann NN, Weissmann N, Muckenthaler MU. The increase in hemoglobin concentration with altitude varies among human populations. Ann N Y Acad Sci. 2019;1450(1):204–20. doi:10.1111/nyas.14136
- Gassmann M, Muckenthaler MU. Adaptation of iron requirement to hypoxic conditions at high altitude. J Appl Physiol. 2015;119(12):1432–40. doi:10.1152/japplphysiol.00248.2015
Rare anemias
Joachim Kunz (Physician Scientist); Andreas Kulozik (Medical Director); Martina Muckenthaler (Professor of Molecular Medicine)
Background
According to the "Global Burden of Disease study" published by the WHO, anemia represents one of the major challenges in child health worldwide. Apart from the common alimentary iron deficiency, anemia is caused by a highly heterogeneous group of more than 90 rare diseases including hereditary abnormalities of globin and heme biosynthesis, membranopathies, and abnormalities of iron metabolism. Following recent large scale migration of people from Africa, the Middle East and Asia to Germany, the prevalence of sickle cell disease and thalassemia has significantly increased thus generating new challenges in this field of rare anemias. This clinically heterogeneous disease is a major challenge for diagnostics and for reaching therapy decisions.
Aims
We recently established a patient registry for "thalassemia and rare anemias" mandated by the GPOH and funded by the Dietmar Hopp Stiftung. Our aim is to assess frequencies of subtypes of this heterogenous disease, provide subclassification, insight into pathological complications as well as adequate treatment options. We further aim to improve diagnostics of unclear anemias and study underlying pathomechanisms in selected cases.
Registry
Registry for thalassemias and other rare anemias.
National/international cooperation
This registry is a successful Germany-wide cooperation with clinical partners in Berlin, Bonn, Freiburg, Koblenz, Munich and Ulm. On the European level we are integrated into RADeep, an initiative of the ERN-EuroBloodNet for the development of european registries for rare anemias. We are further involved as one of the first study hospitals for the Bluebird Bio HGB-212-study that offers gene therapy for the treatment of b-thalassemia major.
Selected publications
- Pasricha S-R, Tye-Din J, Muckenthaler MU, Swinkels D. Clinical Seminar: Iron Deficiency, The Lancet, in pressAltamura S, Vegi NM, Hoppe PS, Schroeder T, Aichler M, Walch A, Okreglicka K, Hültner L, Schneider M, Ladinig C, Kuklik-Roos C, Mysliwietz J, Janik D, Neff F, Rathkolb B, Angelis MTH de, Buske C, Silva ARd, Muedder K, Conrad M, Ganz T, Kopf M, Muckenthaler MU, Bornkamm GW. Glutathione peroxidase 4 and vitamin E control reticulocyte maturation, stress erythropoiesis and iron homeostasis. Haematologica. 2020;105(4):937–50. doi:10.3324/haematol.2018.212977
- Tagliaferri L, Kunz JB, Happich M, Esposito S, Bruckner T, Hübschmann D, Okun JG, Hoffmann GF, Schulz A, Kappe J, Speckmann C, Muckenthaler MU, Kulozik AE. Newborn screening for severe combined immunodeficiency using a novel and simplified method to measure T-cell excision circles (TREC). Clin Immunol. 2017;17551–5. doi: 10.1016/j.clim.2016.11.016
- Greil J, Verga-Falzacappa MV, Echner NE, Behnisch W, Bandapalli OR, Pechanska P, Immenschuh S, Vijayan V, Balla J, Tsukahara H, Schneider M, Janka G, Claus M, Longerich T, Muckenthaler MU, Kulozik AE. Mutating heme oxygenase-1 into a peroxidase causes a defect in bilirubin synthesis associated with microcytic anemia and severe hyperinflammation. Haematologica. 2016;101(11): e436-e439. doi:10.3324/haematol.2016.147090
- Kunz JB, Awad S, Happich M, Muckenthaler L, Lindner M, Gramer G, Okun JG, Hoffmann GF, Bruckner T, Muckenthaler MU, Kulozik AE. Significant prevalence of sickle cell disease in Southwest Germany: results from a birth cohort study indicate the necessity for newborn screening. Ann Hematol. 2016;95(3):397–402. doi:10.1007/s00277-015-2573-y