Research Group Laketa
The projects in our group focus on advanced microscopy infrastructure, microscopy-based assays and their applications in infectious disases research.
Projects
Microscopy and infectious disease research have been inseparable partners ever since in the 1670s Anthonie van Leeuwenhoek used his newly-invented microscope to examine a sample of plaque he had scraped from his own teeth, and observed — for the first time — bacteria and other microorganisms that share the world with us. Undoubtedly, microscopy was fundamental in infectious disease research, as it was necessary for the discovery of infectious agents by direct observation and later on also for their diagnosis.
In modern biomedical research that is being realized in CIID, microscopy-based experimental approach has a central role. A comprehensive understanding of host-pathogen interactions requires quantitative assessment of molecular events across a wide range of spatiotemporal scales and organizational complexities Due to recent technical developments, this is currently only achievable with microscopy. Based on development of new imaging modalities, fluorescent probes and sensors, computer technology, image analysis algorithms and new biological model system, the field of biological microscopy is undergoing a revolution. Only now we are starting to appreciate and make use of the full potential of the microscopy-based experimental approach. We believe that modern microscopy technology is currently uniquely positioned to propel the infectious disease research to a new frontier.
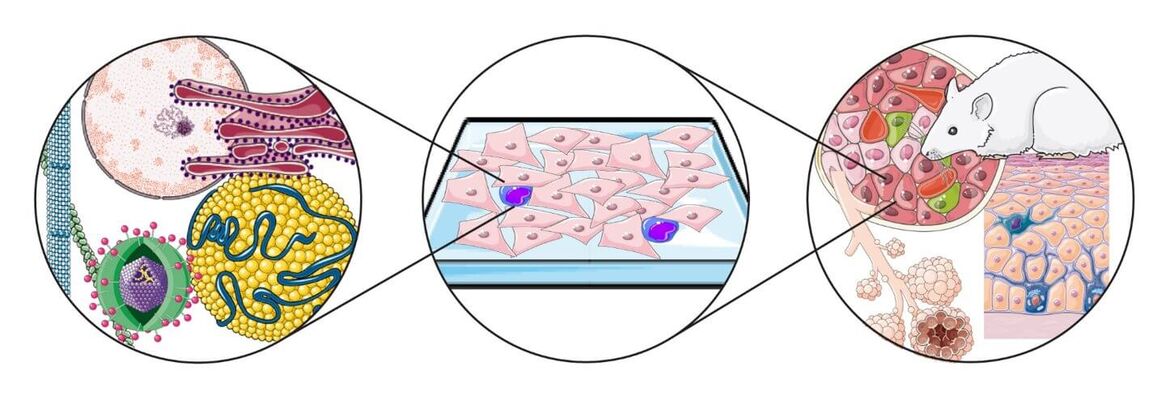
Project 1
Microscopy Infrastructure under BSL2 and BSL3 Containment

The Infectious Disease Imaging Platform (IDIP) represents advanced microscopy and FACS infrastructure under enhanced biosafety containment (BSL2/3) to enable research, drug discovery and diagnostics in the field of infectious diseases. Over 250 m2 of ground (BSL‑2) and underground (BSL‑3) area of the CIID is dedicated to IDIP infrastructure which is organised in 14 interconnected microscopy rooms, tissue culture and sample preparation, image analysis and office areas.
IDIP implements a comprehensive range of bioimaging technologies that allow infectious disease research across vastly different spatiotemporal scales and organizational complexities, from structural studies on the macromolecular scale all the way to whole organ/body live imaging. More information about IDIP organisation and instrumentation can be found on the IDIP homepage. IDIP projects cover all aspects necessary for the execution of the microscopy-based experimental approach in the context of infectious diseases research. Typically, projects are related to the following activities:
- Implementing, operating and providing advanced microscopy and cell sorting infrastructure under enhanced biosafety conditions (BSL‑2 and BSL‑3). You can find more information on IDIP infrastructure here
- Consultation during microscopy-based project planning and experimental design.
- Collaboration within projects being executed in CIID.
- Advice and support in image processing, data visualisation and presentation.
- Development of data acquisition and analysis automation workflows.
- Coordination of microscopy infrastructure investments and support for related grants applications.
- Education and training in microscopy and microscopy-related subjects
Project 2
Development of microscopy-based assays for drug screening and diagnostics
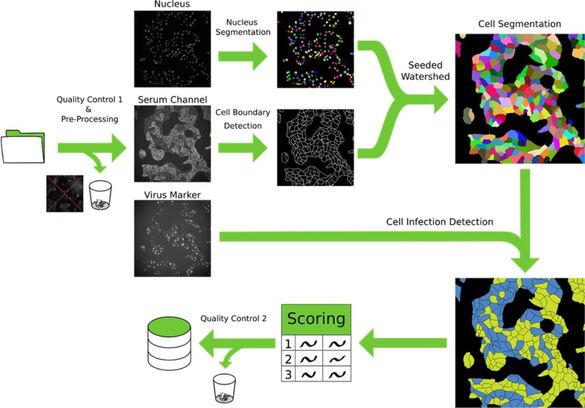
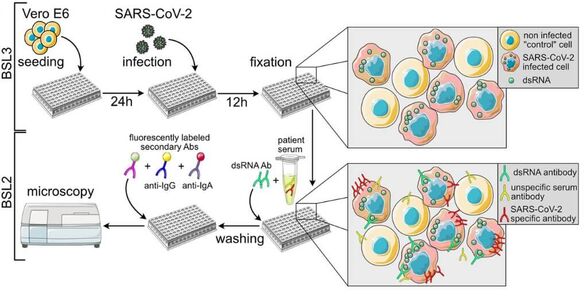
The microscopy-based approach holds immense potential in advancing translational research and revolutionizing drug screening pipelines, as well as enhancing the sensitivity, specificity, and adaptability of medical diagnostic procedures. In light of the continuous emergence of novel pathogens, the development of rapidly adaptable serological and diagnostic assays to assess their impact on human health and society has become indispensable. The advent of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has underscored the ongoing challenge of rapidly evaluating the influence of evolving viral variants on existing immunity, therapeutic options, and vaccines.
To address this challenge and establish a universally applicable methodology for promptly addressing emerging infections in the future, we are currently engaged in the development of an automated multiplex microscopy approach integrated with machine learning-based analysis. By harnessing this innovative technique, we aim to not only to decipher the impact of emerging pathogens on human health but also facilitate the screening and analysis of human therapeutic monoclonal antibodies. We are actively engaged in collaborative initiatives with the CIID and the German Center for Infection Research (Deutsches Zentrum für Infektionsforschung (DZIF)), where we are jointly working on the development of microscopy-based assays addressing diverse projects and a wide range of pathogens.
Selected publications
Complete publication list (PubMed)
- Sartingen N, Stürmer V, Kaltenböck M, Müller TG, Schnitzler P, Kreshuk A, Kräusslich HG, Merle U, Mücksch F, Müller B, Pape C, Laketa V. (2024) Multiplex Microscopy Assay for Assessment of Therapeutic and Serum Antibodies against Emerging Pathogens. Viruses , 16(9), 1473; https://doi.org/10.3390/v16091473
- Sadhu L, Tsopoulidis N, Hasanuzzaman M, Laketa V, Way M, Fackler OT. ARPC5 isoforms and their regulation by calcium-calmodulin-N-WASP drive distinct Arp2/3-dependent actin remodeling events in CD4 T cells. Elife. 2023 May 10;12:e82450. doi: 10.7554/eLife.82450.
- Heuss C, Rothhaar P, Burm R, Lee JY, Ralfs P, Haselmann U, Ströh LJ, Colasanti O, Tran CS, Schäfer N, Schnitzler P, Merle U, Bartenschlager R, Patel AH, Graw F, Krey T, Laketa V, Meuleman P, Lohmann V. A Hepatitis C virus genotype 1b post-transplant isolate with high replication efficiency in cell culture and its adaptation to infectious virus production in vitro and in vivo. PLoS Pathog. 2022 Jun 28;18(6) doi:10.1371/journal.ppat.1010472.
- Cortese M, Laketa V. Advanced microscopy technologies enable rapid response to SARS-CoV-2 pandemic. Cell Microbiol. 2021 Jul;23(7):e13319. doi: 10.1111/cmi.13319
- Klein S, Wimmer WH, Winter SL, Kolovou A, Laketa V, Chlanda P Post-correlation on-lamella cryo-CLEM reveals the membrane architecture of lamellar bodies. Communications Biology, 2021 Jan 29;4(1):137. doi: 10.1038/s42003-020-01567-z
- Müller TG, Zila V, Peters K, Schifferdecker S, Stanic M, Lucic B, Laketa V, Lusic M, Müller B, Kräusslich HG. HIV-1 uncoating by release of viral cDNA from capsid-like structures in the nucleus of infected cells. Elife. 2021 Apr 27;10:e64776. doi:10.7554/eLife.64776
- Tönshoff B, Müller B, Elling R, Renk H, Meissner P, Hengel H, Garbade SF, Kieser M, Jeltsch K, Grulich-Henn J, Euler J, Stich M, Chobanyan-Jürgens K, Zernickel M, Janda A, Wölfle L, Stamminger T, Iftner T, Ganzenmueller T, Schmitt C, Görne T, Laketa V, Olberg S, Plaszczyca A, Cortese M, Bartenschlager R, Pape C, Remme R, Huzly D, Panning M, Weigang S, Giese S, Ciminski K, Ankerhold J, Kochs G, Schwemmle M, Handgretinger R, Niemeyer CM, Engel C, Kern WV, Hoffmann GF, Franz AR, Henneke P, Debatin KM, Kräusslich HG. Prevalence of SARS-CoV-2 Infection in Children and Their Parents in Southwest Germany. JAMA Pediatr. 2021. Jun 1;175(6):586-593. doi: 10.1001/jamapediatrics.2021.0001
- Pape C, Remme R, Wolny A, Olberg S, Wolf S, Cerrone L, Cortese M, Klaus S, Lucic B, Ullrich S, Anders-Össwein M, Wolf S, Cerikan B, Neufeldt CJ, Ganter M, Schnitzler P, Merle U, Lusic M, Boulant S, Stanifer M, Bartenschlager R, Hamprecht FA, Kreshuk A, Tischer C, Kräusslich HG, Müller B, Laketa V. Microscopy-based assay for semi-quantitative detection of SARS-CoV-2 specific antibody levels in human sera. Bioessays. 2021 Mar;43(3):e2000257. doi: 10.1002/bies.202000257.
- Pahmeier F, Neufeldt CJ, Cerikan B, Prasad V, Pape C, Laketa V, Ruggieri A, Bartenschlager R, Cortese M. A Versatile Reporter System To Monitor Virus-Infected Cells and Its Application to Dengue Virus and SARS-CoV-2. J Virol. 2021 Jan 28;95(4):e01715-20. doi: 10.1128/JVI.01715-20K
- Cortese M, Lee JY, Cerikan B, Neufeldt CJ, Oorschot VMJ, Köhrer S, Hennies J, Schieber NL, Ronchi P, Mizzon G, Romero-Brey I, Santarella-Mellwig R, Schorb M, Boermel M, Mocaer K, Beckwith MS, Templin RM, Gross V, Pape C, Tischer C, Frankish J, Horvat NK, Laketa V, Stanifer M, Boulant S, Ruggieri A, Chatel-Chaix L, Schwab Y, Bartenschlager R. Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies. Cell Host Microbe. 2020 Dec 9;28(6):853-866.e5. doi:10.1016/j.chom.2020.11.003
- Bejarano DA, Peng K, Laketa V, Börner K, Jost KL, Lucic B, Glass B, Lusic M, Müller B, Kräusslich HG. HIV-1 nuclear import in macrophages is regulated by CPSF6-capsid interactions at the nuclear pore complex. Elife. 2019 Jan 23;8:e41800. doi: 10.7554/eLife.41800.
- Tsopoulidis N, Kaw S, Laketa V, Kutscheidt S, Baarlink C, Stolp B, Grosse R, Fackler OT. T cell receptor-triggered nuclear actin network formation drives CD4+ T cell effector functions. Sci Immunol. 2019 Jan 4;4(31):eaav1987. doi: 10.1126/sciimmunol.aav1987.
- Laketa V Microscopy in Infectious Disease Research-Imaging Across Scales. J Mol Biol. 2018 Aug 17;430(17):2612-2625. doi: 10.1016/j.jmb.2018.06.018
- Ferrando-May E, Hartmann H, Reymann J, Ansari N, Utz N, Fried HU, Kukat C, Peychl J, Liebig C, Terjung S, Laketa V Sporbert A, Weidtkamp-Peters S, Schauss A, Zuschratter W, Avilov S; German BioImaging network. Advanced light microscopy core facilities: Balancing service, science and career. Microsc Res Tech. 2016 Jun;79(6):463-79. doi: 10.1002/jemt.22648
- Kuchenov D, Laketa V, Stein F, Salopiata F, Klingmüller U, Schultz C. High-Content Imaging Platform for Profiling Intracellular Signaling Network Activity in Living Cells. Cell Chem Biol. 2016 Dec 22;23(12):1550-1559. doi: 10.1016/j.chembiol.2016.11.008
- Laketa V, Zarbakhsh S, Traynor-Kaplan A, Macnamara A, Subramanian D, Putyrski M, Mueller R, Nadler A, Mentel M, Saez-Rodriguez J, Pepperkok R, Schultz C. PIP₃ induces the recycling of receptor tyrosine kinases. Science Signal. 2014 Jan 14;7(308):ra5. doi:10.1126/scisignal.2004532.
- Simpson JC, Joggerst B, Laketa V, Verissimo F, Cetin C, Erfle H, Bexiga MG, Singan VR, Hériché JK, Neumann B, Mateos A, Blake J, Bechtel S, Benes V, Wiemann S, Ellenberg J, Pepperkok R. Genome-wide RNAi screening identifies human proteins with a regulatory function in the early secretory pathway. Nat Cell Biol. 2012 Jun 3;14(7):764-74. doi: 10.1038/ncb2510.
- Laketa V, Simpson JC, Bechtel S, Wiemann S, Pepperkok R. High-content microscopy identifies new neurite outgrowth regulators.Mol Biol Cell. 2007 Jan;18(1):242-52. doi: 10.1091/mbc.e06-08-0666.
Group members
Dr. Vibor Latketa, Head of IDIP
e-mail
CIID Room 029, Phone: +49 6221 56-34410
Dr. Sylvia Olberg, Imaging specialist
e-mail
CIID Room 026, Phone: +49 6221 56-4789
Dr. Severina Klaus, Imaging specialist
e-mail
CIID Room 026, Phone: +49 6221 56-4789
Dr. Jessica Kehrer, Imaging specialist
e-mail
CIID Room 201, Phone: +49 6221 56-32241
Dr. Alessandro Ulivi, Imaging specialist
e-mail
CIID Room 026, Phone: +49 6221 56-4789
Associated experts:
Dr. Bettina Stolp, IDIP associated expert
e-mail
CIID Room 201, Phone: +49 6221 56-54631
Dr. Annett Petrich, IDIP associated expert
e-mail
CIID Room 302, Phone: +49 6221 56-5008
Jana Makroczyova, EM and sample preparation specialist
e-mail
Bioquant, Phone: +49 6221 54-51230
Alumni
Former Group Members
Vanessa Stürmer, Imaging assistant
Dr. Charlotte Kaplan, Super-resolution imaging specialist
Dr. Giulia Mizzon, IDIP associated expert
Dr. Melina Valbracht, IDIP associated expert

