Hepatitis B Virus
4. Early Events in Hepadnaviral Infection
The Duck Hepatitis B Virus Model System
First insights into the hepadnaviral entry mechanisms came from experiments performed with primary duck hepatocytes (PDH) using DHBV, the prototype member of the avian branch of the hepadnaviral family. DHBV can be easily propagated in vivo in its natural host, the Pekin duck. It shows some features that clearly distincts DHBV from HBV. For example, its envelope contains only two proteins, designated L- and S-protein differing in the presence of the 161 amino acids comprising the preS-domain. In infection competition assays, the preS-domain was shown to be responsible for receptor recognition and binding to carboxypeptidase D (CPD), a type-1 transmembrane glycoprotein with a molecular weight of 180kD ( Kuroki et al., 1994; Urban et al., 1998; Breiner et al., 1998 ).
In detailed biochemical studies it was shown that DHBV-preS and dCPD form a complex of extraordinarily high affinity, representing on of the first examples of the preS-domain being an intrinsically unstructured/natively unfolded protein ( Urban et al., 2000 ).
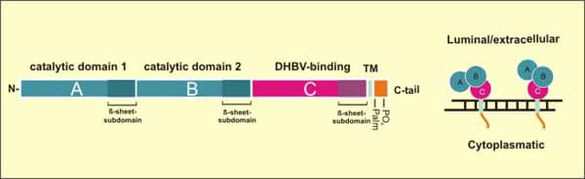
Fig.8: Domain structure of Carboxypeptidase D (CPD). CPD is a trans-Golgi network resident protein cycling to the plasma membrane and back. It is composed of three homologous extracellular/luminal carboxypeptidase-like domains, a transmembrane anchor and a highly conserved cytoplasmatic tail. DHBV binds the enzymatically inactive C-domain near the plasma membrane
Nevertheless, some observations support the assumption that (an) additional factor(s) are (is) required for membrane fusion. Firstly, duCPD alone is not sufficient to transfer infectability when recombinantly expressed in a heterologous cell culture system that supports all intracellular replication steps. Secondly, a distinct sequence N-terminal to the duCPD binding domain was shown to determine host range among avian hepadnaviruses. Our recent discovery of myristoylated preS-derived peptides that interfere with DHBV infection in a CPD-independent manner ( Urban and Gripon, 2002 ) directly supports this hypothesis.
The Human Hepatitis B Virus
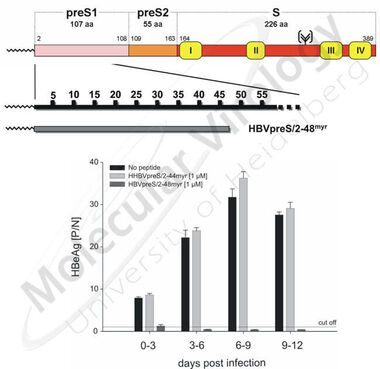
Using primary human hepatocytes it has previously been shown that posttranslational myristoylation of the HBV L-protein prior to its membrane translocation plays a pivotal role for HBV infectivity ( Gripon et al., 1995 ). The same holds true for the integrity of the first 77 preS1 amino acids but not for major parts of preS2 ( LeSeyec et al., 1999 ). Based on these results we could demonstrate that N-terminally myristoylated peptides comprising this sequence (HBVpreS/2-78myr), but also shorter ones (HBVpreS/2-48myr) blocked HBV infection in vitro with remarkable efficacies ( Gripon et al., 2005 , Fig 8) at already picomolar concentrations.
Fig 9. HBV infection competition using myristoylated preS1-derived peptides of the HBV large viral surface protein: At the top the L-protein of HBV is schematically depicted. The synthetic preS1-peptide HBVpreS/2-48myr is enlarged. The bar diagram shows the specific inhibition of HBV infection of HepaRG cells by HBVpreS/2-48myr in comparison to a myristoylated control peptide, derived from the L-protein of the heron hepatitis B virus (HHBVpreS/2-44myr).
We were further able to exactly map the amino acid sequence requirements for infection inhibition, characterize the role of N-terminal acylation and provide evidence that amino acids required for infection inhibition by the peptide are also absolutely essential for HBV and Hepatitis delta virus (HDV) infectivity ( Engelke et al. 2006 ). Using these mutants we are now investigating which step in HBV entry (attachment, endocytosis, fusion) is affected by this region of the HBV preS1-domain. We identify potential HBV-receptors by biochemical and genetic means, study the interaction of these molecules with HBV in vitro and test their specific activity in cell culture assays. We develop efficient transfer systems that allow the expression of foreign genes in hepatocytes. In this way we are able to functionally analyse cellular and viral molecules that participate in the hepadnaviral infection process.
Development of Novel HBV Entry Inhibitors for Therapeutical Use
The remarkable efficacy of preS-derived entry inhibitors of HBV-infection implies possible clinical applications like prophylaxis of HBV-reinfection after liver transplantation or the protection of newborns from vertical HBV-transmission from their infected mothers. Moreover, since it is still unclear to which degree the maintenance of a chronic HBV carrier state depends on constantly ongoing new infection events in the liver (whilst simultaneously infected cells are eliminated by the immune system), entry inhibition might also be an intriguing concept for the treatment of chronic HBV infections.
< Page 3 (Cell Culture System)