Early Clinical Pharmacological Trial Unit (KliPS)
The ISO 9001-certified Clinical Pharmacological Trial Unit (KliPS) plans, coordinates, and conducts early phase (Phase I/II) clinical trials, including first-in-human trials. These trials include not only Investigator-Initiated Trials (IITs), but also sponsored trials, in cooperation with partners from the Center for Internal Medicine and other departments of the hospital as well as with industry partners.
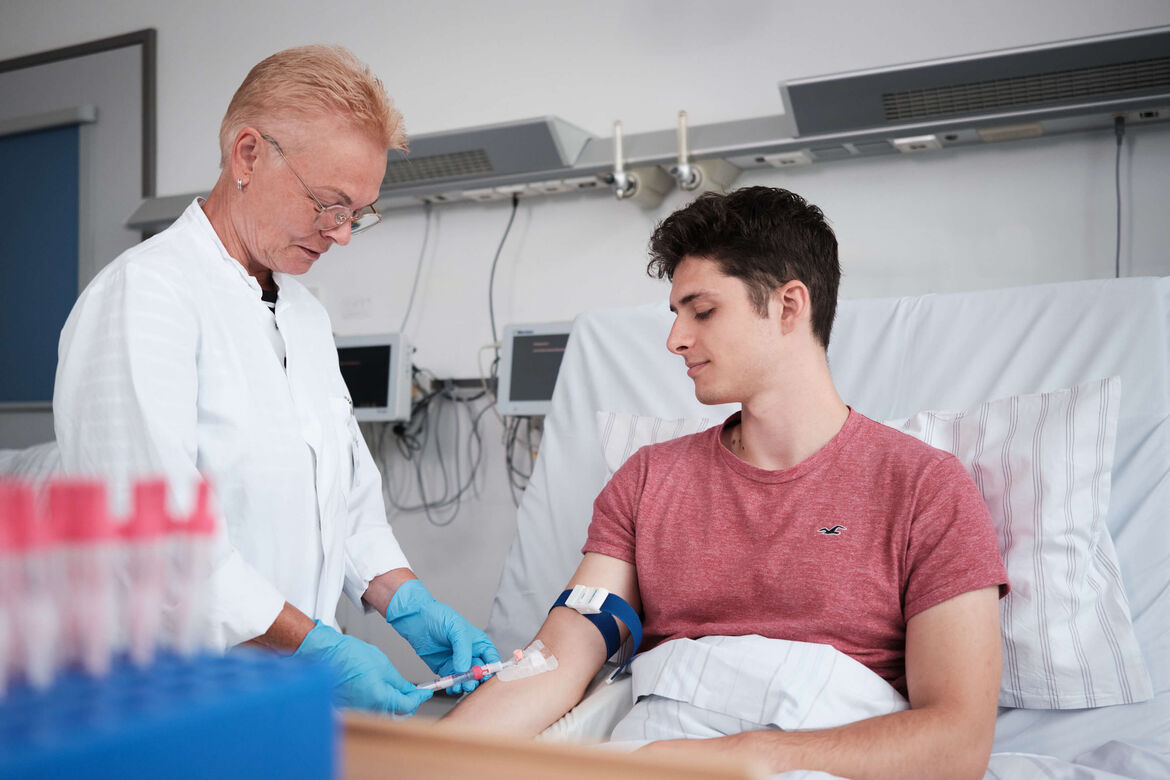
Patients and healthy volunteers are treated in the trial center as inpatients, day patients and outpatients within the framework of trials on various clinical pictures. Phase I trials with healthy volunteers are often the first and necessary step into the clinical development of a new drug. The Heidelberg campus offers an excellent environment for recruiting reliable trial participants.
An experienced team of investigators and study nurses, state-of-the-art equipment and the connection to the Center for Internal Medicine offer an ideal environment for GCP-compliant trials. To date, more than 140 studies have been conducted with a wide variety of drugs (chemical molecules, antibodies and RNA as therapeutics) as well as with therapeutic and preventive vaccines in various indications.
Our Mission
We support the translation of new compounds with our clinical expertise. Through high quality in the planning and conduct of clinical trials, we create safety for trial patients and healthy trial participants and ensure valid results as a basis for a later successful application of the new drug.
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/5/6/csm_willkommen_d34eda0786.png)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/5/csm_ACL_11_GrafikFingerTablet_LB8_c4ce258375.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/3/a/csm_KS_b1_PatBett_Banner_719c5df562.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/6/4/csm_publikaitonen_4abdce50fc.png)