Research Group Translational Modelling
The research group “Translational Modeling” studies and develops methods and models for the quantification of functional and structural changes in vessels and nerve tissue and their extrapolation and integration into clinical routine. We use new techniques in magnetic resonance (MR) imaging such as vessel architectural imaging, arterial spin labeling, relaxometry and mapping techniques at 3 Tesla, 7 Tesla and 9.4 Tesla, as well as high-resolution multiphoton microscopy and light disk fluorescence microscopy in cooperation with the German Cancer Research Center. The resulting quantitative characterization of vascular networks and nerve tissue serves as the basis for the development of functional simulations that examine the changes in the MR signal under changing physiological conditions. The research group combines translational MR-physical basics, statistical image processing, preclinical disease models and clinical applications.
Research focus
- Diffusion effects in MR imaging
- Vascular network architecture and topology
- Microstructural imaging
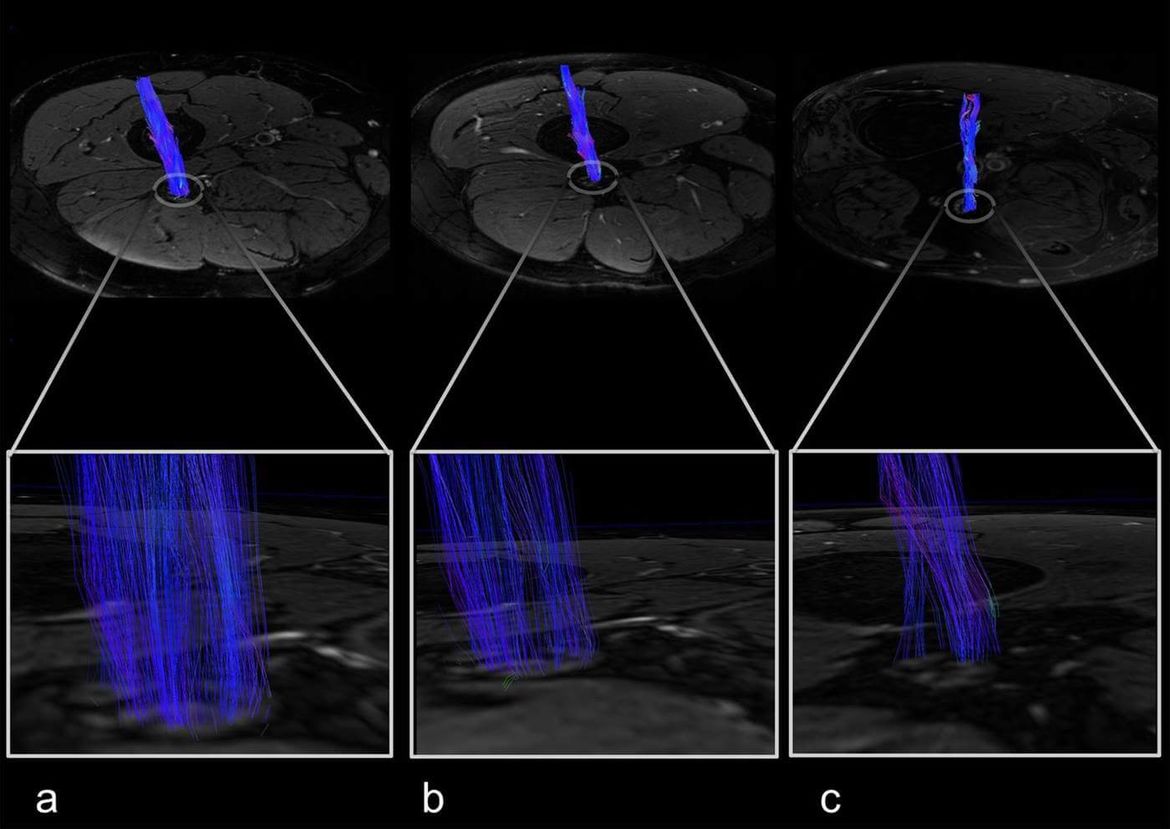
- Quantitative MR imaging of peripheral nerves
- Machine learning
- Statistical modeling
Team
External cooperation
- German Cancer Research Center, Heidelberg (Research groups Experimental Neurooncology, Functional imaging, Pediatric Neurooncology, and Preclinical Imaging)
- Jülich Research Center (Institute of Neuroscience and Medicine)
- University Hospital Würzburg (Institute of Neuroradiology)
- University Hospital Bern (Inselspital), Switzerland
- Johns Hopkins Hospital, Baltimore, USA
- National Institute on Ageing, National Institutes of Health, Bethesda, USA
Internal cooperation
- Department of Radiology, Heidelberg University Hospital
- Department of Endocrinology, Heidelberg University Hospital
- Department of Paraplegiology, Heidelberg University Hospital
- Department of Neurology, Heidelberg University Hospital
Grants and Prizes
- International Foundation for Research in Paraplegia research grant
- DFG grant KU 3555/1-1
- SFB 1158 A03
- 2018 prize of the diffusion study group, International Society of Magnetic Resonance in Medicine
- 2018 poster prize of the German Pain Society (Deutsche Schmerzgesellschaft)
- 2017 Best selected lecture in “Neurodegeneration”, German Society of Neuroradiology (Deutsche Gesellschaft für Neuroradiologie)
- 2014 Physician Scientist Fellowship of the Medical Faculty Heidelberg
- 2014 Hoffmann-Klose-Foundation Stipend
- 2014 Best selected lecture in “Multimodal imaging concepts”, German Society of Neuroradiology (Deutsche Gesellschaft für Neuroradiologie)
Publications
- Dependence of the frequency distribution around a sphere on the voxel orientation. Kurz FT, Buschle LR, Rotkopf LT, Herzog FS, Sterzik A, Schlemmer HP, Kampf T, Bendszus M, Heiland S, Ziener CH. Z Med Phys. 2021;
- Tumor cell plasticity, heterogeneity and resistance in crucial microenvironmental niches in glioma. Jung E, Osswald M, Ratliff M, Dogan H, Xie R, Weil S, Hoffmann D, Kurz FT, Kessler T, Heiland S, Deimling A, Sahm F, Wick W, Winkler F. Nat Comm. 2021;12(1):1014. Doi: 10.1038/s41467-021-21117-3
- Efficient discretization scheme for semi-analytical solutions of the Bloch-Torrey equation. Rotkopf LT, Wehrse E, Kurz FT, Schlemmer HP, Ziener CH. J Magn Reson Open. 2021;6-7: 100010. doi: 10.1016/j.jmro.2021.10001
- Lineshape of magnetic resonance and its effects on free induction decay and steady state free precession signal formation. Ziener CH, Uhrig M, Kampf T, Sturm VJF, Kurz FT, Heiland S, Bendszus M, Pham M, Jakob PM, Schlemmer HP, Buschle LR. Concepts Magn Reson Part A (2020), 5057386. doi: 10.1155/2020/5057386
- Diabetic Polyneuropathy Is Associated With Pathomorphological Changes in Human Dorsal Root Ganglia: A Study Using 3T MR Neurography. Jende JME, Kender Z, Rother C, Alvarez-Ramos L, Groener JB, Pham M, Morgenstern J, Oikonomou D, Hahn A, Juerchott A, Kollmer J, Heiland S, Kopf S, Nawroth PP, Bendszus M, Kurz FT. Front Neurosci. 2020;14:570744. doi: 10.3389/fnins.2020.570744.
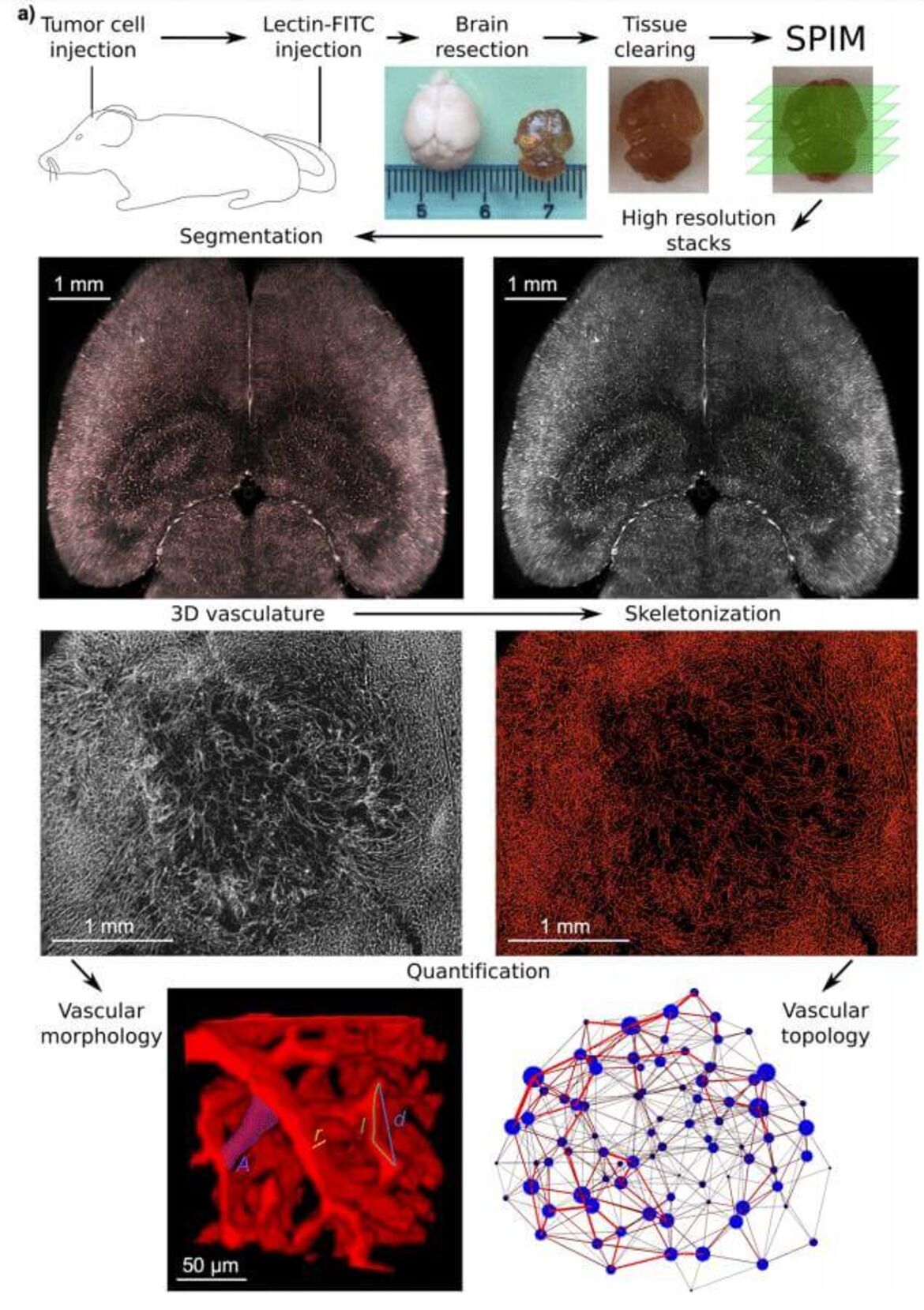
- Large-scale characterization of the microvascular geometry in development and disease by tissue clearing and quantitative ultramicroscopy. Hahn A, Bode J, Alexander A, Karimian-Jazi K, Schregel K, Schwarz D, Sommerkamp AC, Krüwel T, Abdollahi A, Wick W, Platten M, Bendszus M, Tews B, Kurz FT, Breckwoldt MO.J Cereb Blood Flow Metab. 2020:271678X20961854. doi: 10.1177/0271678X20961854. Online ahead of print.
- Comparison of non-contrast-enhanced dental magnetic resonance imaging and cone-beam computed tomography in assessing the horizontal and vertical components of furcation defects in maxillary molars: An in vivo feasibility study. Juerchott A, Sohani M, Schwindling FS, Jende JME, Kurz FT, Rammelsberg P, Heiland S, Bendszus M, Hilgenfeld T.J Clin Periodontol. 2020;47(12):1485-1495. doi: 10.1111/jcpe.13374.
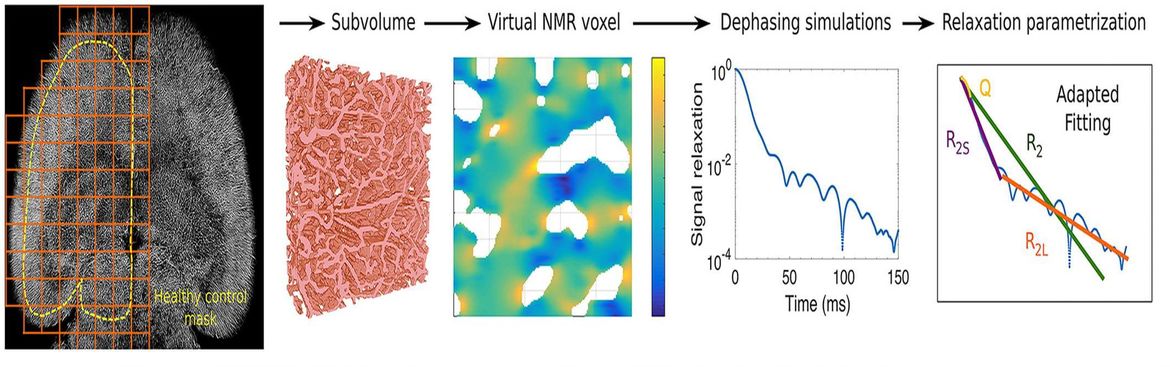
- Brain tumor classification of virtual NMR voxels based on realistic blood vessel-induced spin dephasing using support vector machines.Hahn A, Bode J, Schuhegger S, Krüwel T, Sturm VJF, Zhang K, Jende JME, Tews B, Heiland S, Bendszus M, Breckwoldt MO, Ziener CH, Kurz FT. NMR Biomed. 2020:e4307. doi: 10.1002/nbm.4307.
- Diabetes Increases the Vulnerability of the Cardiac Mitochondrial Network to Criticality.Vetter L, Cortassa S, O'Rourke B, Armoundas AA, Bedja D, Jende JME, Bendszus M, Paolocci N, Sollot SJ, Aon MA, Kurz FT. Front Physiol. 2020;11:175.
- Gibbs point field model quantifies disorder in microvasculature of U87-glioblastoma.Hahn A, Bode J, Krüwel T, Kampf T, Buschle LR, Sturm VJF, Zhang K, Tews B, Schlemmer HP, Heiland S, Bendszus M, Ziener CH, Breckwoldt MO, Kurz FT. J Theor Biol. 2020;494:110230.
- In vivo accuracy of dental magnetic resonance imaging in assessing maxillary molar furcation involvement: a feasibility study in humans.Juerchott A, Sohani M, Schwindling FS, Jende JME, Kurz FT, Rammelsberg P, Heiland S, Bendszus M, Hilgenfeld T. J Clin Periodontol. 2020;47(7):809-815. doi: 10.1111/jcpe.13299.
- Jende JME, Groener J, Kender Z, Hahn A, Morgenstern J, Heiland S, Nawroth P, Bendszus M, Kopf S, Kurz FT. Troponin T parallels structural nerve damage in type 2 diabetes – a cross-sectional study using magnetic resonance neurography. Diabetes. 2020;69(4):713-723. doi: 10.2337/db19-1094.
- Jende JME, Groener JB, Kender Z, Rother C, Hahn A, Hilgenfeld T, Juerchott A, Preisner F, Heiland S, Kopf S, Nawroth P, Bendszus M, Kurz FT. Structural nerve remodeling on 3-T MR neurography differs between painful and painless diabetic polyneuropathy in either type 1 or type 2 diabetes. Radiology. 2020;294(2):405-414. doi: 10.1148/radiol.2019191347.
- Karimian-Jazi K, Munch P, Alexander A, Fischer M, Piechutta M, Karreman MA, Solecki GM, Berghoff AS, Pfleiderer K, Friedrich M, Deumelandt K, Kurz FT, Wick W, Heiland S, Bendszus M, Winkler F, Platten M, Breckwoldt MO. Monitoring innate immune cell dynamics in the glioma microenvironment by correlated magnetic resonance imaging and multiphoton microscopy (MR-MPM). Theranostics. 2020;10(4):1873-1883. doi: 10.7150/thno.38659.
- Groener JB, Jende JME, Kurz FT, Kender Z, Treede RD, Schuh-Hofer S, Nawroth PP, Bendszus M, Kopf S. Understanding diabetic neuropathy: from subclinical nerve lesions to severe nerve fiber deficits. A cross-sectional study in patients with type 2 diabetes and healthy controls. Diabetes. 2020;69(3):436-447. doi: 10.2337/db19-0197.
- Buschle LR, Kurz FT, Schlemmer HP, Ziener CH. Neumann-Weber integral transform for complex indices. J Math Phys. 2019; 60:043502.
- Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, Körber C, Kardorff M, Ratliff M, Xie R, Horstmann H, Messer M, Paik SP, Knabbe J, Sahm F, Kurz FT, Acikgöz AA, Herrmannsdörfer F, Agarwal A, Bergles DE, Chalmers A, Miletic H, Turcan S, Mawrin C, Hänggi D, Liu HK, Wick W, Winkler F, Kuner T. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019; 573(7775):532-538.
- Glioblastoma multiforme restructures the topological connectivity of cerebrovascular networks. Hahn A, Bode J, Krüwel T, Solecki G, Heiland S, Bendszus M, Tews B, Winkler F, Breckwoldt MO, Kurz FT. Sci Rep. 2019;13;9(1):11757.
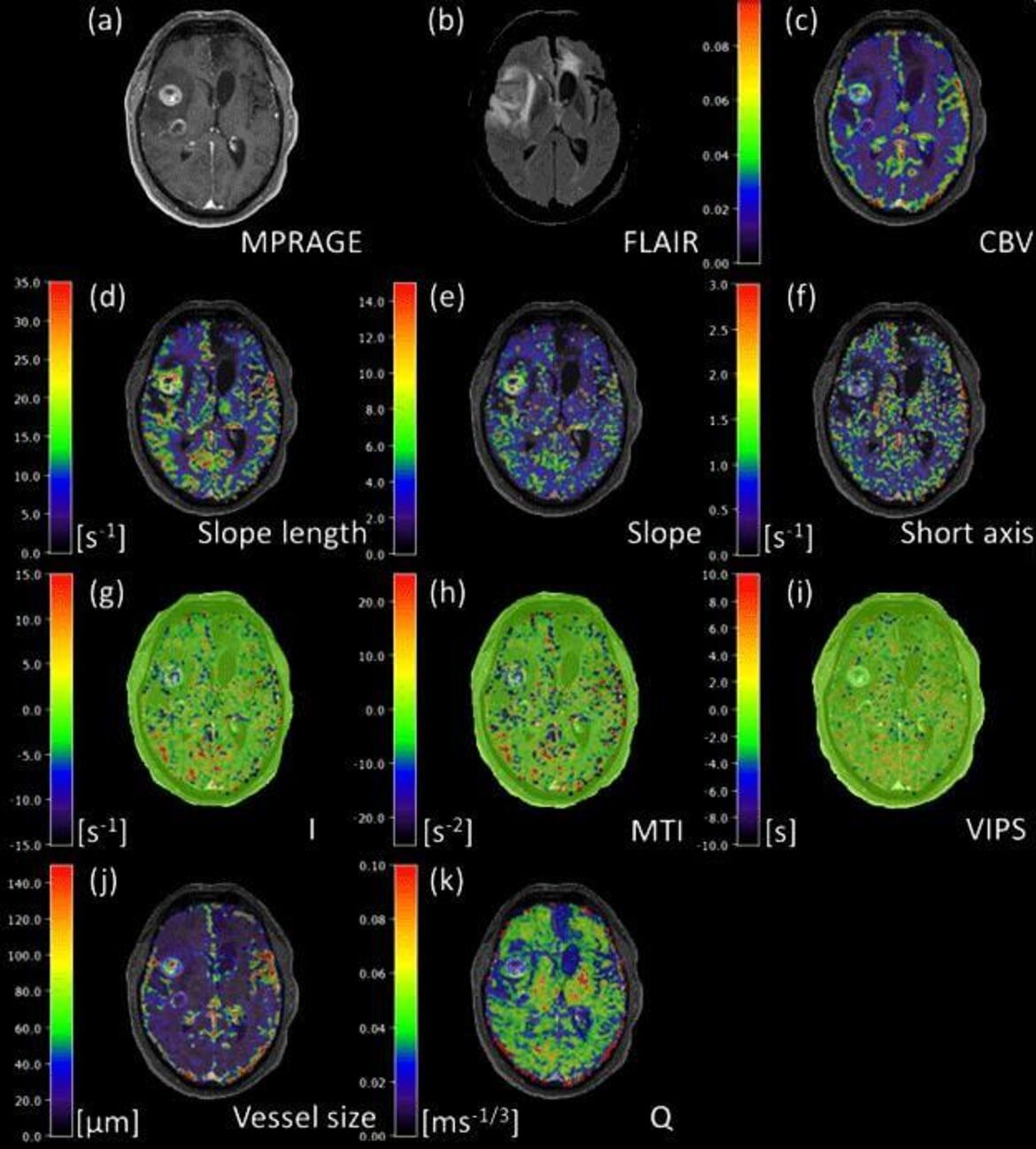
- Vessel architecture imaging using multiband gradient-echo/spin-echo EPI. Zhang K, Yun SD, Triphan SMF, Sturm VJ, Buschle LR, Hahn A, Heiland S, Bendszus M, Schlemmer HP, Shah NJ, Ziener CH, Kurz FT. PLoS One. 2019;9;14(8):e0220939.
- Association of Serum Cholesterol Levels With Peripheral Nerve Damage in Patients With Type 2 Diabetes. Jende JME, Groener JB, Rother C, Kender Z, Hahn A, Hilgenfeld T, Juerchott A, Preisner F, Heiland S, Kopf S, Pham M, Nawroth P, Bendszus M, Kurz FT. JAMA Netw Open. 2019;3;2(5):e194798.
- Correlated MRI and Ultramicroscopy (MR-UM) of Brain Tumors Reveals Vast Heterogeneity of Tumor Infiltration and Neoangiogenesis in Preclinical Models and Human Disease. Breckwoldt MO, Bode J, Sahm F, Krüwel T, Solecki G, Hahn A, Wirthschaft P, Berghoff AS, Haas M, Venkataramani V, von Deimling A, Wick W, Herold-Mende C, Heiland S, Platten M, Bendszus M, Kurz FT, Winkler F, Tews B. Front Neurosci. 2019;10;12:1004.
- Lineshape of magnetic resonance and its effects on free induction decay and steady state free precession signal formation. Ziener CH, Uhrig M, Kampf T, Sturm VJF, Kurz FT, Heiland S, Bendszus M, Pham M, Jakob PM, Schlemmer HP, Buschle LR. Concept Magn Reson B. 2019. In press.
- Improved compressed sensing reconstruction for F magnetic resonance imaging. Kampf T, Sturm VJF, Basse-Lüsebrink TC, Fischer A, Buschle LR, Kurz FT, Schlemmer HP, Ziener CH, Heiland S, Bendszus M, Pham M, Stoll G, Jakob PM. MAGMA. 2019; 32(1):63-77.
- Voxel-size dependent quantitative susceptibility mapping of blood vessel networks: a simulation study. Buschle LR, Kampf T, Kurz FT, Sturm VJF, Pham M, Schlemmer HP, Ziener CH. Z Med Phys. 2019;29(3):282-291.
- Spin dephasing around randomly distributed vessels. Buschle LR, Kurz FT, Kampf T, Schlemmer HP, Ziener CH. J Magn Reson. 2019;299:12-20.
- Pseudo-diffusion effects in lung MRI. Ziener CH, Kampf T, Kurz FT, Schlemmer HP, Buschle LR. J Magn Reson. 2019;299:1-11.
- Dual-contrast pCASL using simultaneous gradient-echo/spin-echo multiband EPI. Zhang K, Sturm VJ, Buschle LR, Hahn A, Yun SD, Jon Shah N, Bendszus M, Heiland S, Schlemmer HP, Ziener CH, Kurz FT. Magn Reson Imaging. 2019;57:359-367.
- Diffusion effects in myelin sheath free induction decay. Kurz FT, Buschle LR, Hahn A, Jende JME, Bendszus M, Heiland S, Ziener CH. J Magn Reson. 2018;297:61-75.
- Dependence of the frequency distribution around a vessel on the voxel orientation. Buschle LR, Kampf T, Kurz FT, Vogel P, Piekarek F, Sturm VJF, Pham M, Schlemmer HP, Ziener CH. Magn Reson Imaging. 2019;57:259-270.
- Assessing Spatiotemporal and Functional Organization of Mitochondrial Networks. Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Methods Mol Biol. 2018;1782:383-402.
- A PRDX1-p38α heterodimer amplifies MET-driven invasion of IDH-wildtype and IDH-mutant gliomas. Wirthschaft P, Bode J, Simon AEM, Hoffmann E, van Laack R, Krüwel T, Dietrich F, Bucher D, Hahn A, Sahm F, Breckwoldt MO, Kurz FT, Hielscher T, Fischer B, Dross N, Ruiz de Almodovar C, von Deimling A, Herold-Mende C, Plass C, Boulant S, Wiestler B, Reifenberger G, Lichter P, Wick W, Tews B. Int J Cancer. 2018;143(5):1176-1187.
- Vessel radius mapping in an extended model of transverse relaxation. Buschle LR, Ziener CH, Zhang K, Sturm VJF, Kampf T, Hahn A, Solecki G, Winkler F, Bendszus M, Heiland S, Schlemmer HP, Kurz FT. MAGMA. 2018;31(4):531-551.
- Diabetic neuropathy differs between type 1 and type 2 diabetes: Insights from magnetic resonance neurography. Jende JME, Groener JB, Oikonomou D, Heiland S, Kopf S, Pham M, Nawroth P, Bendszus M, Kurz FT. Ann Neurol. 2018;83(3):588-598.
- Tweety-Homolog 1 Drives Brain Colonization of Gliomas. Jung E, Osswald M, Blaes J, Wiestler B, Sahm F, Schmenger T, Solecki G, Deumelandt K, Kurz FT, Xie R, Weil S, Heil O, Thomé C, Gömmel M, Syed M, Häring P, Huber PE, Heiland S, Platten M, von Deimling A, Wick W, Winkler F. J Neurosci. 2017;37(29):6837-6850.
- Functional Implications of Cardiac Mitochondria Clustering. Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Adv Exp Med Biol. 2017;982:1-24.
- The influence of spatial patterns of capillary networks on transverse relaxation. Kurz FT, Ziener CH, Rückl M, Hahn A, Sturm VJF, Zhang K, Buschle LR, Bendszus M, Heiland S, Schlemmer HP, Bauer WR, Kampf T. Magn Reson Imaging. 2017;40:31-47.
- Dephasing and diffusion on the alveolar surface. Buschle LR, Kurz FT, Kampf T, Wagner WL, Duerr J, Stiller W, Konietzke P, Wünnemann F, Mall MA, Wielpütz MO, Schlemmer HP, Ziener CH. Phys Rev E. 2017;95(2-1):022415.
- Spin dephasing in a magnetic dipole field around large capillaries: Approximative and exact results. Kurz FT, Buschle LR, Kampf T, Zhang K, Schlemmer HP, Heiland S, Bendszus M, Ziener CH. J Magn Reson. 2016;273:83-97.
- Generalized moment analysis of magnetic field correlations for accumulations of spherical and cylindrical magnetic perturbers. Kurz FT, Kampf T, Buschle LR, Schlemmer HP, Bendszus M, Heiland S, Ziener CH. Front Phys 2016;4:46.
- Network dynamics: quantitative analysis of complex behavior in metabolism, organelles, and cells, from experiments to models and back. Kurz FT, Kembro JM, Flesia AG, Armoundas AA, Cortassa S, Aon MA, Lloyd D. Wiley Interdiscip Rev Syst Biol Med. 2017;9(1).
- CPMG relaxation rate dispersion in dipole fields around capillaries. Kurz FT, Kampf T, Buschle LR, Heiland S, Schlemmer HP, Bendszus M, Ziener CH. Magn Reson Imaging. 2016;34(7):875-88.
- Mitochondrial redox and pH signaling occurs in axonal and synaptic organelle clusters. Breckwoldt MO, Armoundas AA, Aon MA, Bendszus M, O'Rourke B, Schwarzländer M, Dick TP, Kurz FT. Sci Rep. 2016;6:23251.
- Experimental Cerebral Malaria Spreads along the Rostral Migratory Stream. Hoffmann A, Pfeil J, Alfonso J, Kurz FT, Sahm F, Heiland S, Monyer H, Bendszus M, Mueller AK, Helluy X, Pham M. PLoS Pathog. 2016;12(3):e1005470.
- Assessment of tumor oxygenation and its impact on treatment response in bevacizumab-treated recurrent glioblastoma. Bonekamp D, Mouridsen K, Radbruch A, Kurz FT, Eidel O, Wick A, Schlemmer HP, Wick W, Bendszus M, Østergaard L, Kickingereder P. J Cereb Blood Flow Metab. 2017;37(2):485-494.
- [Principles and applications of susceptibility weighted imaging]. Kurz FT, Freitag M, Schlemmer HP, Bendszus M, Ziener CH. Radiologe. 2016;56(2):124-36.
- Correlated magnetic resonance imaging and ultramicroscopy (MR-UM) is a tool kit to assess the dynamics of glioma angiogenesis. Breckwoldt MO, Bode J, Kurz FT, Hoffmann A, Ochs K, Ott M, Deumelandt K, Krüwel T, Schwarz D, Fischer M, Helluy X, Milford D, Kirschbaum K, Solecki G, Chiblak S, Abdollahi A, Winkler F, Wick W, Platten M, Heiland S, Bendszus M, Tews B. Elife. 2016;5:e11712.
- Microstructural Analysis of Peripheral Lung Tissue through CPMG Inter-Echo Time R2 Dispersion. Kurz FT, Kampf T, Buschle LR, Schlemmer HP, Heiland S, Bendszus M, Ziener CH. PLoS One. 2015;10(11):e0141894.
- Diffusion propagators for hindered diffusion in open geometries. Ziener CH, Kampf T, Kurz FT. Concepts in Magn Reson Part A 2015;44:150-159.
- Brain tumour cells interconnect to a functional and resistant network. Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, Huang L, Ratliff M, Karimian Jazi K, Kurz FT, Schmenger T, Lemke D, Gömmel M, Pauli M, Liao Y, Häring P, Pusch S, Herl V, Steinhäuser C, Krunic D, Jarahian M, Miletic H, Berghoff AS, Griesbeck O, Kalamakis G, Garaschuk O, Preusser M, Weiss S, Liu H, Heiland S, Platten M, Huber PE, Kuner T, von Deimling A, Wick W, Winkler F. Nature. 2015;528(7580):93-8.
- Orthogonality, Lommel integrals and cross product zeros of linear combinations of Bessel functions. Ziener CH, Kurz FT, Buschle LR, Kampf T. Springerplus. 2015;4:390.
- Diffusion-mediated dephasing in the dipole field around a single spherical magnetic object. Buschle LR, Kurz FT, Kampf T, Triphan SMF, Schlemmer HP, Ziener CH. Magn Reson Imaging. 2015;33(9):1126-1145.
- Mitochondrial networks in cardiac myocytes reveal dynamic coupling behavior. Kurz FT, Derungs T, Aon MA, O'Rourke B, Armoundas AA. Biophys J. 2015;108(8):1922-33.
- Free induction decay caused by a dipole field. Ziener CH, Kurz FT, Kampf T. Phys Rev E. 2015;91(3):032707.
- Cardiac mitochondria exhibit dynamic functional clustering. Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Front Physiol. 2014;5:329.
- Theoretical model of the single spin-echo relaxation time for spherical magnetic perturbers. Kurz FT, Kampf T, Heiland S, Bendszus M, Schlemmer HP, Ziener CH. Magn Reson Med. 2014;71(5):1888-95.






